Additional Treatment Using Transcatheter Arterial Infusion with Drug-Eluting Beads Transarterial Chemoembolization Contributes to Prolonged Survival of Patients with BCLC Stage C Hepatocellular Carcinoma after Discontinuing Lenvatinib: Preliminary Study
A B S T R A C T
Objective: Lenvatinib is considered the first-line treatment for unresectable advanced hepatocellular carcinoma (HCC); however, in some clinical cases, discontinuation of lenvatinib is unavoidable. It is important to elucidate if transcatheter arterial infusion (TAI) with drug-eluting beads transarterial chemoembolization (DEB-TACE) is a feasible second-line treatment after discontinuing lenvatinib. In this study, we aimed to evaluate the efficacy, hepatic function and nutritional status associated with TAI with DEB-TACE for patients who previously discontinued lenvatinib.
Materials and Methods: We included 35 patients who were prescribed lenvatinib for unresectable HCC between July 2018 and December 2019, of whom 12 discontinued lenvatinib during the study. The changes in the albumin-bilirubin (ALBI) score and the controlling nutritional status (CONUT) score before and after discontinuing lenvatinib were examined. Furthermore, the tolerability and survival of patients treated using TAI with DEB-TACE as a second-line treatment were analysed.
Results: The ALBI and CONUT scores were significantly worse when lenvatinib was started and stopped (p<0.05). The CONUT score was significantly worse in the second-line group than in the follow-up group when beginning and discontinuing lenvatinib; however, this score tended to improve after DEB-TACE. The group that underwent TAI with DEB-TACE as a second-line treatment had significantly better survival than the follow-up group (log-rank test, p=0.029; generalized Wilcoxon test, p=0.042).
Conclusion: In patients who could undergo TAI with DEB-TACE as a second-line treatment after discontinuing lenvatinib, the CONUT score improved, while the ALBI score was maintained and well-tolerated; these scores may have contributed to improved survival compared with follow-up patients. Future studies with larger sample sizes are necessary to confirm our findings.
Keywords
Hepatocellular carcinoma, lenvatinib, transcatheter arterial infusion, drug-eluting beads transarterial, chemoembolization, controlling nutritional status score, albumin-bilirubin (ALBI) score
Introduction
Hepatocellular carcinoma (HCC) is one of the worst leading causes of cancer death globally [1]. Advanced HCC has a poor prognosis and limited treatment options are available. Recent studies have reported a new type of systemic therapy that is available. Sorafenib has long been used as first-line systemic chemotherapy, while lenvatinib (LEN) was approved in March 2018 [2]. A previous study revealed that LEN is non-inferior to sorafenib as first-line chemotherapy and is now widely used [3]. However, there are some cases in which treatment is discontinued, even with LEN. It is necessary to consider what kind of treatment is appropriate, as well as the treatment indications in patients who have discontinued LEN.
Transcatheter arterial infusion (TAI) and transcatheter arterial chemoembolization (TACE) are alternative and locoregional therapies for unresectable HCC. We previously reported that whole-liver TAI with a high concentration of a fine-powder formulation of cisplatin (DDP-H) (IA-call®; Nippon Kayaku Co. Ltd., Tokyo, Japan) prior to radical local treatment inhibited the intrahepatic recurrence of HCC [4]. Additionally, survival outcomes were improved in HCC patients with a Japan Integrated Staging score between 0 and 1 who underwent whole-liver TAI with DDP-H; hepatic function was not impaired in these patients [5]. TACE is classified into conventional TACE (cTACE), balloon-TACE, and drug-eluting bead (DEB)-TACE [6]. DEB-TACE is approved, even for Child B, and is considered second-line treatment. DEB-TACE involves the selective application of chemotherapy-loaded microbeads, which embolize tumor arteries and ensure that the loaded chemotherapeutic agent is slowly released; this achieves a lower systemic drug peak compared to cTACE [7].
The aim of the present study was to evaluate the efficacy and nutritional function associated with the use of TAI with DEB-TACE and DDP-H as a second-line treatment after the discontinuation of LEN in Barcelona Clinic Liver Cancer (BCLC) stage C HCC patients.
Methods
I Study Design and Patients
This single-center, explorative, cohort study included 12 patients with HCC who had previously discontinued LEN between June 2019 and December 2019. Five patients received TAI with DEB-TACE as a second-line treatment. Seven patients were followed-up. We included patients who 1) were diagnosed with a tumor stage equivalent to Barcelona Clinic Liver Cancer (BCLC) stage C, 2) were observed for at least 14 days, 3) had no other advanced cancer complications, 4) were administered LEN previously as a systemic treatment, and 5) had an Eastern Cooperative Oncology Group score of 0 or 1. On the other hand, we excluded patients who 1) had BCLC stage A or B, and 2) were unable to be followed-up according to the schedule. HCC was diagnosed using dynamic contrast-enhanced computed tomography or dynamic contrast-enhanced magnetic resonance imaging.
II Assessment of Immuno-Nutritional Status and Hepatic Functional Reserve
The CONUT scores, used to assess immuno-nutritional status, were calculated from the following three parameters, as previously described: 1) albumin levels of 3.5, 3.0-3.49, 2.5-2.99, and <2.5 g/dL were scored as 0, 2, 4, and 6 points, respectively; 2) total lymphocyte counts of 1600, 1200-1599, 800-1199, and <800/L were scored as 0, 1, 2, and 3 points, respectively; 3) total cholesterol levels of 180, 140-79, 100-139, and <100 mg/dL were scored as 0, 1, 2, and 3 points, respectively [8]. Separately, the Child-Pugh classification and ALBI grade were used to assess hepatic functional reserve [9, 10].
III Treatment
In the five patients for whom TAI with DEB-TACE was administered as a second-line treatment, the femoral artery was punctured using the Seldinger technique, and a 5-F introducer was inserted, followed by the insertion of a 5-F catheter. Subsequently, a 3-F microcatheter was advanced into the proper hepatic artery, and 65 mg/m2 of DDP-H (IA-call®, Nippon Kayaku, Co., Ltd.) was infused via the catheter into the whole liver over 30 min. The DDP-H solution was prepared by dissolving DDP-H (100 mg/vial) in 70 ml of physiological saline that had previously been warmed to 50°C (cisplatin concentration: 1.43 mg/ml). A 5-hydroxytryptamine-3 receptor antagonist and corticosteroids were administered during DDP-H infusion to reduce emesis. To prevent renal toxicity, hydration was performed via an intravenous drip infusion of fluids (500 ml prior to DDP-H infusion and 1,500 ml following DDP-H infusion), and the patients were instructed to consume ≥2,000 ml/day of water beginning the day after treatment.
Following DDP‑H infusion, DEB-TACE was administered to embolize subsectional and/or peripheral nutrient vessels. The patients were treated with an intra-arterial injection of 1-2 g DEB (HepaSphere, Nippon Kayaku, Co. Ltd, Japan). The size of DEB varied from 100 µm to 500 µm, and the amount of epirubicin (Farmorubicin; Pfizer Japan, Tokyo, Japan) used ranged from 30 mg to 50 mg. The embolization endpoint was defined as static blood flow in the tumor-feeding artery, which was often measured by the time it took to clear the contrast column (typically four heartbeats). In all procedures, DEB-TACE was performed until static blood flow was observed in the target artery.
Adverse events were graded according to the National Cancer Institute 69 General Terminology for Adverse Events (NCI-CTCAE, version 4.0). Treatment was usually discontinued because of a Grade 4 clinical adverse event. Analyses included changes in the albumin-bilirubin (ALBI) score at the start of LEN treatment, the time when LEN was discontinued, and after beginning the second-line treatment; changes in the controlling nutritional status (CONUT) score at the time of LEN discontinuation and after second-line treatment were also analysed [8, 10].
IV Statistical Analysis
Overall survival (OS) was calculated using the Kaplan-Meier method and differences were analysed using the log-rank test. The comparison between the two groups was determined by Chi-square test; normally distributed continuous data were presented as mean ± standard deviation, and the comparison between the two groups was determined by t-test. The differences in parameters were analysed using one-way measures ANOVA.
Statistical significance was defined as p<0.05. All statistical analyses were performed using EZR (Saitama Medical Centre, Jichi Medical University, Shimotsuke, Japan), a graphical user interface for R version 3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria) [11].
Results
Table 1 shows data for cases that discontinued LEN. The 12 patients comprised were five males and seven females, with an average age of 76.5 years (range, 67-86 years). All patients discontinued LEN owing to adverse events. The etiologies of liver diseases included hepatitis C (determined by positivity for the hepatitis C virus [HCV] antibody) in nine (75.0%) patients, hepatitis B (as determined by positivity for the hepatitis B virus [HBV] surface antigen) in one (8.3%) patient and neither HCV or HBV in two (16.7%) patients. The baseline Child-Pugh score was 5 in three cases (25.0%) and 6 in nine cases (75.0%). The median follow-up period for all 12 patients was 202 days (range, 20-430 days). An average of 2.5 repeated treatments were performed in the second-line group. The ALBI and CONUT scores were significantly worse when LEN was started and stopped (p<0.05) (Table 2). Furthermore, there were no significant differences in any background clinical parameters between the second-line group and the follow-up group (Table 3).
Table 1: Baseline characteristics after the discontinuation of lenvatinib for HCC patients.
|
Characteristic |
n=12 |
|
Mean age (years, range) |
76.5 ±7.465 (67-86) |
|
Sex (male/female) |
6/6 |
|
Etiology (HBV/HCV/NBNC) |
1/9/2 |
|
Child-Pugh score (5/6) |
4/8 |
|
ALBI grade (1/2) |
4/8 |
|
BCLC (B/C) |
0/12 |
|
CONUT score (normal/mild/moderate/severe) |
2/4/4/2 |
|
Portal vein tumor thrombosis (present/absent) |
4/8 |
|
Extrahepatic spread (present/absent) |
4/8 |
|
AFP (ng/mL) |
140.72±56.58 |
|
DCP (mAU/mL) |
448.20±210.36 |
HCC: Hepatocellular Carcinoma; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; NBNC: Non-HBV and Non-HCV; ALBI: Albumin-Bilirubin; BCLC: Barcelona Cancer Liver Clinic; CONUT: Controlling Nutritional Status; AFP: Alpha-Fetoprotein; DCP: Des-Gamma Carboxy-Prothrombin.
Table 2: Changes in ALBI score and CONUT score at baseline when starting lenvatinib and after discontinuing lenvatinib.
|
|
Baseline |
LEN stop |
p-value |
|
ALBI score |
−2.15±0.61 |
−1.67±0.51 |
p<0.05. |
|
CONUT score |
3.73±2.90 |
6.27±2.87 |
P<0.05 |
ALBI: Albumin-Bilirubin; CONUT: Controlling Nutritional Status; LEN: Lenvatinib.
Table 3: Baseline characteristics of TAI with DEB-TACE in the second-line group or in the follow-up group after lenvatinib treatment.
|
|
Second line |
Follow up |
p-value |
|
Mean age (years, range) |
77.00±7.45 |
76.14±7.46 |
p=0.7838 |
|
Sex (male/female) |
3/2 |
3/4 |
p=0.5582 |
|
Etiology (HBV/HCV/NBNC) |
1/4/0 |
0/5/2 |
p=0.2397 |
|
Child-Pugh score (5/6) |
2/3 |
3/4 |
p=0.9212 |
|
ALBI grade (1/2) |
2/3 |
3/4 |
p=0.9212 |
|
BCLC (B/C) |
0/5 |
0/7 |
p=1.000 |
|
Portal vein tumor thrombosis (present/absent) |
2/3 |
3/4 |
p=0.9212 |
|
Extrahepatic spread (present/absent) |
2/3 |
3/4 |
p=0.9212 |
|
AFP (ng/mL) |
193.20±92.87 |
110.35±71.33 |
p=0.4285 |
|
DCP (mAU/mL) |
801.22±417.63 |
127.27±68.04 |
p=0.1113. |
HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; NBNC: Non-HBV and Non-HCV; ALBI: Albumin-Bilirubin; BCLC: Barcelona Cancer Liver Clinic; CONUT: Controlling Nutritional Status; AFP: Alpha-Fetoprotein; DCP: Des-Gamma Carboxy-Prothrombin; n.s.: not significant.
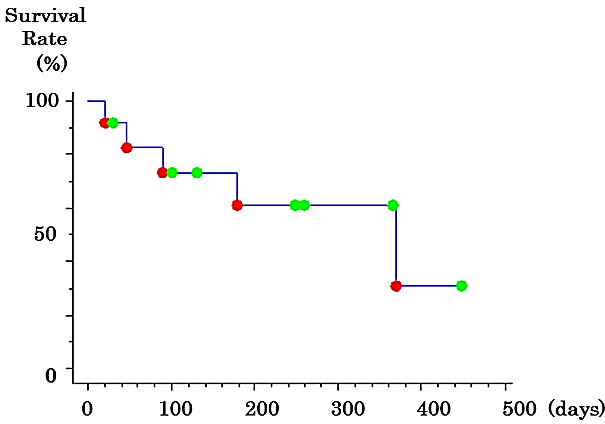
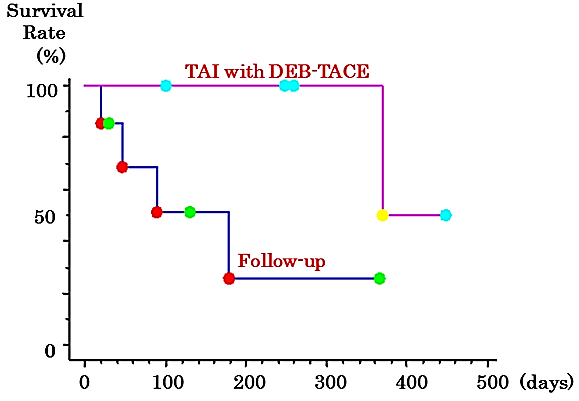
In the second-line group, the CONUT score was significantly worse than that in the follow-up group when beginning and discontinuing LEN; however, it tended to improve after DEB-TACE. Although the ALBI score also deteriorated temporarily, it tended to improve after TACE (Table 4). On the other hand, in the follow-up group, the CONUT and ALBI scores deteriorated over time (Table 5). The overall 50% survival time after LEN discontinuation was 369 days (Figure 1). The group that underwent DEB-TACE as a second-line treatment had a significantly higher survival time compared to the follow-up group (log-rank test, p=0.029; generalized Wilcoxon test, p=0.042) (Figure 2).
Figure 1: Kaplan-Meier survival curve for the patients with advanced hepatocellular carcinoma who discontinued lenvatinib.
Table 4: Changes in the ALBI and CONUT scores at baseline, when starting and discontinuing lenvatinib, and after TAI with DEB-TACE in the second-line group.
|
Parameter |
Baseline |
LEN stop |
After second-line |
|
ALBI score |
−2.26±0.1 |
−1.6±0.68 |
−1.88±0.34 |
|
CONUT score |
2.75±2.22 |
7.0±0.82* |
5.0±0.82* |
Parameter are described as mean±SD at baseline, LEN stop and after second line. The differences in parameters were analyzed using a one-way measures ANOVA. * p=0.01.
ALBI: Albumin-Bilirubin; CONUT: Controlling Nutritional Status; LEN: Lenvatinib; DEB-TACE: Drug-Eluting Beads Transarterial Chemoembolization; TAI: Transcatheter Arterial Infusion.
Table 5: Changes in the ALBI and CONUT scores at baseline, when starting and discontinuing lenvatinib in the follow-up group.
|
Parameter |
Baseline |
LEN stop |
After follow up |
|
ALBI score |
−2.03±0.74 |
−1.61±0.48 |
−1.57±1.14 |
|
CONUT score |
4.14±3.44 |
5.37±3.46 |
6.29±4.39 |
Parameter are described as mean ± SD at baseline, LEN stop and after follow up.
ALBI: Albumin-Bilirubin; CONUT: Controlling Nutritional Status; LEN: Lenvatinib.
Figure 2: Kaplan-Meier survival curve for the patients with advanced hepatocellular carcinoma who discontinued lenvatinib and underwent TAI with DEB-TACE as a second-line treatment.
DEB-TACE: Drug-Eluting Beads Transarterial Chemoembolization; TAI: Transcatheter Arterial Infusion.
There were no occurrences of treatment-related mortality in the second-line group. Treatment-related adverse events were assessed according to the National Cancer Institute Common Terminology Criteria, version 4.0. Adverse events were evaluated as the maximum change in the grade within one month of therapy. Grade 3 or 4 adverse events did not occur in either group. Grade 1 or 2 adverse events occurred in the second-line group only. These events included an elevated aspartate aminotransferase (AST) level in two patients (40.0%), an elevated alanine aminotransferase (ALT) level in three (60.0%), thrombocytopenia in one (20.0%) and hyperbilirubinemia in one patient (20.0%). Regardless of the use of platinum, grade 3 or 4 renal dysfunction was not observed in either group.
Discussion
The purpose of this study was to evaluate the contribution of TAI with DEB-TACE as a second-line treatment for unresectable BCLC stage C HCC patients after the discontinuation of LEN and its clinical benefits, in terms of nutrition changes. Among the current treatment strategies for advanced HCC, LEN is one of the best first-line treatments, with a high therapeutic response and agents that target progression-free survival [3]. However, regardless of the efficacy of LEN as a molecular targeting agent, some patients discontinued LEN due to the risk of adverse events. Therefore, the treatment period is limited in patients who discontinue LEN. Currently, there are no known studies reporting the use of second-line therapies after discontinuing LEN.
To evaluate the long-term survival after LEN treatment, various treatments are often administered in the form of multidisciplinary therapy. HCC patients might exhibit molecular heterogeneity and a nonuniform baseline population. Therefore, multidisciplinary therapy might have some impact on the treatment of HCC patients with various disease stages. However, the efficacy of multidisciplinary treatment in patients who fail to respond to LEN remains elusive. Combination treatment with sorafenib and cisplatin (CDDP) powder tended to improve the overall survival (OS) in advanced HCC patients compared to sorafenib alone [12]. Regarding subsequent second-line molecular-targeted drugs, previous clinical trials have failed to show any efficacy in HCC patients who failed to respond to sorafenib. In clinical practices, other alternative options, including hepatic arterial infusion chemotherapy, are often administered as a second-line treatment option for patients who have failed to respond to sorafenib treatment [13, 14].
We believe that transcatheter methods, such as TAI and TACE, also play an important role in the treatment of advanced HCC. We previously reported that TAI with a high concentration of a fine-powder formulation of cisplatin (DDP-H) reduced intrahepatic HCC recurrence and improved survival [4, 5]. TACE is the standard therapy for BCLC classification intermediate stage B HCC [15]. Furthermore, cTACE, DEB-TACE, and balloon-occluded TACE are alternative strategies for unresectable HCC. TAI with B-TACE contributes to improved survival of patients with BCLC stage B HCC [16]. However, the combined use of TAI with DDP-H and DEB-TACE for BCLC stage C HCC patients discontinuing LEN has not yet been investigated.
Recently, immuno-nutritional status has been reported to be associated with outcomes in patients with HCC [17, 18]. The controlling nutritional status (CONUT) score has been developed as an objective assessment tool of nutritional status [8]. CONUT is scored from 0 to 8 points, and 5 points is considered a malnutrition status [15]. The CONUT score is based on three variables: serum albumin level, total cholesterol level, and total lymphocyte count [15]. The CONUT score is now used as an immuno-nutritional index [19, 20]. Although the CONUT score has previously been reported to improve the prognosis of LEN treatment, it was similar after discontinuing LEN in our study. DEB-TACE has been reported to have fewer effects on the hepatic reserve than cTACE, and a recent study revealed that it was effective even with Child B [21, 22]. In addition, DEB-TACE was able to repeat the treatment to a certain extent, which enabled control of the disease state; because the CONUT score can be maintained, repeated treatment was possible, which may have contributed to the prognosis.
In the present study, TAI with DEB-TACE was administered as second-line targeted therapy in five patients (41.6%). The survival time was significantly prolonged in the TAI and DEB-TACE group compared with that in the follow-up group. Furthermore, in terms of nutritional status, the CONUT score tended to improve in the TAI with DEB-TACE group after discontinuing LEN. Our study is the first known study to demonstrate that interventions consisting of subsequent second-line or additional treatment options after the discontinuation of LEN are associated with a longer OS for HCC patients with BCLC stage C.
This study has some limitations that should be addressed. First, the study was in retrospective nature; therefore, only patients who were expected to have a longer prognosis, whose liver functions were retained or whose disease conditions had not worsened, and who received subsequent or additional treatment, were included. Second, the sample size was relatively small. To obtain more concrete conclusions, future studies should include a larger number of patients treated using second-line therapies after the discontinuation of LEN. Furthermore, it might be necessary to establish the efficacy associated with methylthioamphetamine (MTA) drugs. However, this study suggested that HCC patients who had discontinued LEN were candidates for subsequent or additional treatment options, such as TAI with DEB-TACE, in clinical practices.
In conclusion, the findings of the present study need to be confirmed in larger clinical trials, but the results we found can aid in determining the prognosis of HCC patients who have discontinued lenvatinib and undergo a second-line therapy. TAI with DEB-TACE as a second-line therapy had significantly better CONUT and ALBI scores than the follow-up group. Furthermore, the second-line group exhibited improved overall survival. This preliminary study needs to be confirmed in larger clinical trials.
Ethical Statements
This study was approved by the Institutional Review Board of Saiseikai Niigata Hospital and was conducted according to the principles of the Declaration of Helsinki. All patients provided written informed consent before participating in the study.
Conflicts of Interest
None.
Funding
None.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 07, Aug 2020Accepted: Thu 20, Aug 2020
Published: Mon 31, Aug 2020
Copyright
© 2023 Toru Ishikawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.08.28
Author Info
Toru Ishikawa Saori Endo Michitaka Imai Motoi Azumi Yujiro Nozawa Tomoe Sano Akito Iwanaga Terasu Honma Toshiaki Yoshida
Corresponding Author
Toru IshikawaDepartment of Gastroenterology and Hepatology, Saiseikai Niigata Hospital, Niigata, Japan
Figures & Tables
Table 1: Baseline characteristics after the discontinuation of lenvatinib for HCC patients.
|
Characteristic |
n=12 |
|
Mean age (years, range) |
76.5 ±7.465 (67-86) |
|
Sex (male/female) |
6/6 |
|
Etiology (HBV/HCV/NBNC) |
1/9/2 |
|
Child-Pugh score (5/6) |
4/8 |
|
ALBI grade (1/2) |
4/8 |
|
BCLC (B/C) |
0/12 |
|
CONUT score (normal/mild/moderate/severe) |
2/4/4/2 |
|
Portal vein tumor thrombosis (present/absent) |
4/8 |
|
Extrahepatic spread (present/absent) |
4/8 |
|
AFP (ng/mL) |
140.72±56.58 |
|
DCP (mAU/mL) |
448.20±210.36 |
HCC: Hepatocellular Carcinoma; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; NBNC: Non-HBV and Non-HCV; ALBI: Albumin-Bilirubin; BCLC: Barcelona Cancer Liver Clinic; CONUT: Controlling Nutritional Status; AFP: Alpha-Fetoprotein; DCP: Des-Gamma Carboxy-Prothrombin.
Table 2: Changes in ALBI score and CONUT score at baseline when starting lenvatinib and after discontinuing lenvatinib.
|
|
Baseline |
LEN stop |
p-value |
|
ALBI score |
−2.15±0.61 |
−1.67±0.51 |
p<0.05. |
|
CONUT score |
3.73±2.90 |
6.27±2.87 |
P<0.05 |
ALBI: Albumin-Bilirubin; CONUT: Controlling Nutritional Status; LEN: Lenvatinib.
Table 3: Baseline characteristics of TAI with DEB-TACE in the second-line group or in the follow-up group after lenvatinib treatment.
|
|
Second line |
Follow up |
p-value |
|
Mean age (years, range) |
77.00±7.45 |
76.14±7.46 |
p=0.7838 |
|
Sex (male/female) |
3/2 |
3/4 |
p=0.5582 |
|
Etiology (HBV/HCV/NBNC) |
1/4/0 |
0/5/2 |
p=0.2397 |
|
Child-Pugh score (5/6) |
2/3 |
3/4 |
p=0.9212 |
|
ALBI grade (1/2) |
2/3 |
3/4 |
p=0.9212 |
|
BCLC (B/C) |
0/5 |
0/7 |
p=1.000 |
|
Portal vein tumor thrombosis (present/absent) |
2/3 |
3/4 |
p=0.9212 |
|
Extrahepatic spread (present/absent) |
2/3 |
3/4 |
p=0.9212 |
|
AFP (ng/mL) |
193.20±92.87 |
110.35±71.33 |
p=0.4285 |
|
DCP (mAU/mL) |
801.22±417.63 |
127.27±68.04 |
p=0.1113. |
HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; NBNC: Non-HBV and Non-HCV; ALBI: Albumin-Bilirubin; BCLC: Barcelona Cancer Liver Clinic; CONUT: Controlling Nutritional Status; AFP: Alpha-Fetoprotein; DCP: Des-Gamma Carboxy-Prothrombin; n.s.: not significant.
Table 4: Changes in the ALBI and CONUT scores at baseline, when starting and discontinuing lenvatinib, and after TAI with DEB-TACE in the second-line group.
|
Parameter |
Baseline |
LEN stop |
After second-line |
|
ALBI score |
−2.26±0.1 |
−1.6±0.68 |
−1.88±0.34 |
|
CONUT score |
2.75±2.22 |
7.0±0.82* |
5.0±0.82* |
Parameter are described as mean±SD at baseline, LEN stop and after second line. The differences in parameters were analyzed using a one-way measures ANOVA. * p=0.01.
ALBI: Albumin-Bilirubin; CONUT: Controlling Nutritional Status; LEN: Lenvatinib; DEB-TACE: Drug-Eluting Beads Transarterial Chemoembolization; TAI: Transcatheter Arterial Infusion.
Table 5: Changes in the ALBI and CONUT scores at baseline, when starting and discontinuing lenvatinib in the follow-up group.
|
Parameter |
Baseline |
LEN stop |
After follow up |
|
ALBI score |
−2.03±0.74 |
−1.61±0.48 |
−1.57±1.14 |
|
CONUT score |
4.14±3.44 |
5.37±3.46 |
6.29±4.39 |
Parameter are described as mean ± SD at baseline, LEN stop and after follow up.
ALBI: Albumin-Bilirubin; CONUT: Controlling Nutritional Status; LEN: Lenvatinib.
DEB-TACE: Drug-Eluting Beads Transarterial Chemoembolization; TAI: Transcatheter Arterial Infusion.
References
- Villanueva A (2019) Hepatocellular carcinoma. N Engl J Med 380: 1450-1462. [Crossref]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E et al. (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378-390. [Crossref]
- Kudo M, Finn RS, Qin S, Han KH, Ikeda K et al. (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 391: 1163-1173. [Crossref]
- Ishikawa T, Higuchi K, Kubota T, Seki K, Honma T et al. (2011) Prevention of Intrahepatic Distant Recurrence by Transcatheter Arterial Infusion Chemotherapy with Platinum Agents for Stage I/II Hepatocellular Carcinoma. Cancer 117: 4018-4025. [Crossref]
- Ishikawa T, Kubota T, Abe S, Watanabe Y, Sugano T et al. (2014) Hepatic Arterial Infusion Chemotherapy with Cisplatin Before Radical Local Treatment of Early Hepatocellular Carcinoma (JIS Score 0/1) Improves Survival. Ann Oncol 25: 1379-1384. [Crossref]
- Yang B, Liang J, Qu Z, Yang F, Liao Z et al. (2020) Transarterial Strategies for the Treatment of Unresectable Hepatocellular Carcinoma: A Systematic Review. PLoS One 15: e0227475. [Crossref]
- Varela M, Real MI, Burrel M, Forner A, Sala M et al. (2007) Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 46: 474-481. [Crossref]
- Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B et al. (2005) CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 20: 38-45. [Crossref]
- Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D et al. (2005) Systematic review: The model for end-stage liver disease-Should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther 22: 1079-1089. [Crossref]
- Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M et al. (2015) Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach‐the ALBI grade. J Clin Oncol 33: 550-558. [Crossref]
- Kanda Y (2013) Investigation of the Freely Available Easy-To-Use Software 'EZR' for Medical Statistics. Bone Marrow Transplant 48: 452-458. [Crossref]
- Ikeda M, Shimizu S, Sato T, Morimoto M, Kojima Y et al. (2016) Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol 27: 2090-2096. [Crossref]
- Terashima T, Yamashita T, Arai K, Sunagozaka H, Kitahara M et al. (2014) Feasibility and efficacy of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma after sorafenib. Hepatol Res 44: 1179-1185. [Crossref]
- Shao YY, Liang PC, Wu YM, Huang CC, Huang KW et al. (2013) A pilot study of hepatic arterial infusion chemotherapy for patients with advanced hepatocellular carcinoma who have failed antiangiogenic therapy. Liver Int 33: 1413-1419.
- European Association for the Study of the Liver (2018) EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69: 182-236. [Crossref]
- Ishikawa T, Abe S, Watanabe T, Nozawa Y, Sano T et al. (2016) Improved survival with double platinum therapy transcatheter arterial infusion using cisplatin and transcatheter arterial chemoembolization using miriplatin for BCLC-B hepatocellular carcinoma. Mol Clin Oncol 5: 511-516. [Crossref]
- Ji F, Liang Y, Fu S, Chen D, Cai X et al. (2017) Prognostic value of combined preoperative prognostic nutritional index and body mass index in HCC after hepatectomy. HPB (Oxford) 19: 695-705. [Crossref]
- Peng W, Li C, Wen TF, Yan LN, Li B et al. (2016) Postoperative prognostic nutritional index change is an independent predictor of survival in patients with small hepatocellular carcinoma. Am J Surg 212: 122-127. [Crossref]
- Elghiaty A, Kim J, Jang WS, Park JS, Heo JE et al. (2019) Preoperative controlling nutritional status (CONUT) score as a novel immune-nutritional predictor of survival in non-metastatic clear cell renal cell carcinoma of </= 7 cm on preoperative imaging. J Cancer Res Clin Oncol 145: 957-965. [Crossref]
- Hirahara N, Tajima Y, Fujii Y, Kaji S, Kawabata Y et al. (2019) Controlling Nutritional Status (CONUT) as a prognostic immunonutritional biomarker for gastric cancer after curative gastrectomy: A propensity score-matched analysis. Surg Endosc 33: 4143-4152. [Crossref]
- Shimose S, Kawaguchi T, Iwamoto H, Tanaka M, Miyazaki K et al. (2020) Controlling nutritional status (CONUT) score is associated with overall survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib: A Multicenter Cohort Study. Nutrients 12: 1076. [Crossref]
- Kloeckner R, Weinmann A, Prinz F, Pinto dos Santos D, Ruckes C et al. (2015) Conventional Transarterial Chemoembolization Versus Drug-Eluting Bead Transarterial Chemoembolization for the Treatment of Hepatocellular Carcinoma. BMC Cancer 10: 465. [Crossref]
