Acute Pancreatitis in Child with Relapsing Hepatitis A Viral Infection
A B S T R A C T
Acute pancreatitis in association with acute viral hepatitis A is uncommon. We report a 12-year-old boy with acute pancreatitis associated with relapsing hepatitis A viral infection who made a satisfactory recovery.
Keywords
Viral hepatitis, pancreatitis, children
Introduction
Viral A hepatitis is a self-limited infection occurring predominantly among children usually as an anicteric often subclinical illness. Although hepatitis viruses have a strong tropism for hepatocytes, viral antigens have also been detected in other tissues such as pancreas and gallbladder [1-3]. Several viral infections have been implicated as an etiological factor of acute pancreatitis. The viruses most frequently responsible are the mumps virus, Coxsackie B virus, Epstein Barr virus [1-3]. Acute pancreatitis has been reported very rarely in acute non fulminant viral hepatitis [4-6]. The objective of this report is present a patient with acute pancreatitis associated with acute hepatitis caused by hepatitis A virus (HAV) not at in acute phase, who made a satisfactory recovery.
Brief Report
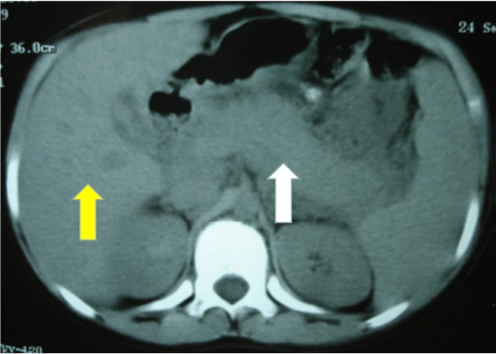
A 12-year-old boy with a previous medical history due to tiredness, numerous episodes of vomiting, dark urine, jaundiced in 2005 July in a little-town in Brazil. A diagnosis of acute viral hepatitis A was made with appearance of HAV IgM antibodies. However, in 2005 September this patient was guided to the Infectious Emergency Department with nausea, jaundice, abdominal pain and vomiting. On admission, he was afebrile and jaundiced. His abdomen was distended with general tenderness on palpation and low bowel sounds were heard. The liver was enlarged 8 cm below the right margin. Laboratory investigations included: white blood count 12.8 X 109/L (71% Polymorphs); Hemoglobin 12,9 g/dL; serum amylase: 795 U/dL (normal 25-115); serum lipase: 958 U/dL (normal 144-286) Bilirubin: 25,6 mg/dL (normal – 0.2–1.2) (Conjugated – 20.9); aspartate aminotransferase (AST): 637 U/L (normal 15-37); alanine aminotransferase (ALT): 751 U/L (normal 30-65); alkaline phosphatase: 310 U/L (normal – 50-136); Gama Glutamiltransferase (GGT): 100 U/L (normal – 5-85). The plasma electrolytes, serum lipid profile, creatinine and calcium are normal. Computed tomography (CT) abdomen showed features of acute edematous of the pancreas body, without calcifications (Figure 1) and enlarged liver.
Figure 1: Computed tomography (CT) abdomen showed features of acute edematous of the pancreas (White Arrow) body, without calcifications and enlarged liver (Yellow Arrow).
Treatment was with fluid and electrolyte replacement. Pain ceased eight days after admission. He regained his appetite three days after. On discharged AST was 211 U/L, ALT 209 U/L, serum amylase 135 U/dL, serum lipase 115 U/dL, bilirubin: 7,4 mg/dL (normal – 0.2–1.2) (Conjugated – 4, 5), alkaline phosphatase 157 U/L, GGT 286 U/L. The patient had normal exams eight weeks after the diagnosis, and until today he didn’t have any disease or relapse. The diagnosis of pancreatitis in our patient was based on clinical features of vomiting, severe abdominal pain, CT abdomen evidence of pancreatitis, elevated serum amylase and lipase and lack of other recognizable abdominal disease.
Discussion
Etiological association of viruses with acute pancreatitis is well known [1-3]. The association of pancreatitis with non-fulminant hepatitis is rare and has been described, predominantly in man [4-9]. The most of patients reported had presented with symptomatic pancreatitis in the early phase of the hepatic illness, and just two patients reported by Garty et al. and Agarwal et al. in the other period of the illness like our patient [7, 9]. Pancreatitis was diagnosed on the basis of typical prolonged pain, high serum amylase and lipase, CT findings. Pancreatitis was not severe in the reported cases in eight children, as well as in our patient [4-9]. Most cases of acute pancreatitis complicating acute viral hepatitis have occurred in conjunction with fulminant hepatitis [10, 11]. In the autopsy series of Ham and Fitzpatrick, 14 (33%) of 42 patients with fulminant hepatic failure, the majority viral etiology, had histologic pancreatitis ranging in severity from mild (five patients) to severe (four patients) [10]. Parbhoo et al. reported similar findings; 21 (36%) of 59 patients with fulminant hepatic failure, which was viral in 42 patients, had pancreatitis at autopsy [11]. In a prospective survey, the same authors found that 14 (23%) of 60 patients with fulminant hepatic failure had elevation of serum amylase [11].
The mechanism of pancreatitis in patients with acute viral hepatitis (non-fulminant) is unknown and it may be multifactorial. One proposed pathogenesis of pancreatitis associate with hepatitis is the development of edema of the ampulla of Vater with obstruction to the outflow of pancreatic fluid [3]. There is no direct evidence for the routes by which hepatitis viruses reach the pancreas; however, the proposed routes are most likely blood or bile. A more plausible mechanism for viral associated acute pancreatitis acinar membrane is direct inflammation and destruction of pancreatic acinar cell by the virus [12]. Acute pancreatitis occurs in 5,65% of patients with acute viral hepatitis, it is mild and recovers with conservative management [4]. Prolonged cholestasis and relapsing hepatitis are well described [13, 14]. Cholestasis will spontaneously resolve but may predispose the patient to develop a relapse of the hepatitis. A biphasic or relapsing form of viral hepatitis A occurs in 5 to 20% of cases [13, 14]. The initial episode lasts 3 to 5 weeks and is followed by a period of remission characterized by normal liver chemistries lasting 4 to 7 weeks. Relapse may mimic the initial episode of the acute hepatitis, that may be the cause of acute pancreatitis in our patient.
The patient had a mild to moderate pancreatitis without any local systemic complication and had uneventful recovery from both hepatitis and pancreatitis, with six and eight weeks after the admission respectively. However, laboratory findings were normal in some 5 to 10 days in other cases [4-8] in pancreatitis, our case need more time to recover, but hepatitis laboratory findings was similar recovery. We did not find any correlation of pancreatitis and or hyperamylasemia and hyperlipasemia to either bilirubin, AST/ALT levels like others that was found by Parbhoo et al. [4-9, 11]. The etiology of pancreatitis was considered to be due to a hepatitis virus inasmuch as there was no evidence of gallstone, alcoholism, drugs, trauma or metabolic causes. Although biliary microscopy was not performed, the fact that there was no recurrence of pancreatitis and no development of gallstone/sludge on follow up (36 months) exclude biliary microlithiasis as a possible etiology.
In summary, acute pancreatitis should always be considered when acute or disproportionate abdominal pain complicates acute viral hepatitis. Management of acute viral pancreatitis remains conservative, with which treatment our patient recovered.
Article Info
Article Type
Case ReportPublication history
Received: Sat 20, Jun 2020Accepted: Wed 01, Jul 2020
Published: Sat 04, Jul 2020
Copyright
© 2023 Álvaro Pimenta Dutra. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JCMCR.2020.02.04
Author Info
Álvaro Pimenta Dutra Fernando Filizzola Mattos Inácio Roberto de Carvalho Suzana Fonseca de Oliveira Melo Talitah Michel Sanchez Candian
Corresponding Author
Álvaro Pimenta DutraGeneral Pediatric Service of the João Paulo II Children’s Hospital/FHEMIG, (Hospital Infantil João Paulo II/Fundação Hospitalar de Minas Gerais), Brazil
Figures & Tables
References
- K Naficy, R Nategh, H Ghadimi (1973) Mumps pancreatitis without parotitis. Br Med J 1: 529. [Crossref]
- Wislocki LC (1973) Acute Pancreatitis in infectious mononucleosis. NEJM 275: 322.
- C Y Tsui, G E Burch, J M Harb (1972) Pancreatitis in a mice infected with Coxsackie B infection. Arch Pathol 93: 379-389. [Crossref]
- Pankaj Jain, Sandeep Nijhawan, Ramesh Roop Rai, Subhash Nepalia, Amit Mathur (2007) Acute pancreatitis in acute viral hepatitis. World J Gastroenterol 13: 5741-5744. [Crossref]
- A Lopez Morante, C Rodriguez de Lope, G San Miguel, F Pons Romero (1986) Acute pancreatitis in hepatitis A infection. Postgrad Med J 62: 497-408. [Crossref]
- L A Shrier, S J Karpen, C McEvoy (1995) Acute pancreatitis associated with acute hepatitis A in a young child. J Pediatr 126: 57-59. [Crossref]
- B Z Garty, D Kanner, Y L Danon (1995) Pancreatitis associated with hepatitis A viral infection. J Pediatr 127: 669. [Crossref]
- A Mishra, S Saigal, R Gupta, S K Sarin (1999) Acute Pancreatitis with viral hepatits: a report of six cases and review of literature. Amn J Gastroenterol 94: 2292-2295. [Crossref]
- K S Agarwal, J M Puliyel, A Mathew, D Lahoti, R Gupta (1999) Acute pancreatitis with cholestatic hepatitis: an unusual manifestation of hepatitis A. Ann Trop Paediatr 19: 391-394. [Crossref]
- J M Ham, P Fitzpatrick (1973) Acute pancreatitis in patients with acute hepatic failure. Am J Dig Dis 18: 1079-1083. [Crossref]
- S P Parbhoo, J Welch, S Sherlock (1973) Acute pancreatitis in patients with fulminant hepatic failure. Gut 14: 428. [Crossref]
- D M Parenti, W Steinberg, P Kang (1996) Infectious causes of acute pancreatitis. Pancreas 13: 356-371. [Crossref]
- E R Schiff (1992) Atypical clinical manifestations of hepatitis A. Vaccine 10: S18-S20. [Crossref]
- M Glikson, E Galun, R Oren, R Tur Kaspa, D Shouval (1992) Relapsing hepatitis A. Review of 14 cases and literature survey. Medicine 71: 14-23. [Crossref]
