Acute Esophageal Necrosis (AEN) - A Rare Syndrome: Report of a Case in a Patient with an Underlying Malignancy and Review of the Literature
A B S T R A C T
Acute Esophageal Necrosis (AEN) is a rare medical disorder characterized by a diffuse circumferential black esophageal mucosa. The majority of patients present with signs of upper gastrointestinal bleeding. Diagnosis is based on esophagogastroduodenoscopy. Treatment consists of intravenous fluids, proton pomp inhibitors and treatment of the underlying illness. We report a case of acute necrotizing oesophagitis (AEN) with an underlying malignancy.
Keywords
Acute esophageal necrosis, cancer, radiotherapy, chemotherapy
Introduction
Acute esophageal necrosis (AEN), also known as black esophagus and necrotizing esophagitis, is a severe form of acute esophagitis which endoscopy shows a dark, black-appearing color (“black esophagus”) [1-3]. Is a particularly rare syndrome with an incidence of less than 0.1 percent in endoscopic series and appears to be more than four times higher in men as compared with women [3-5]. It is uniquely characterized by the univesal involvement of the distal esophagus to the gastroesophageal junction.
The etiology of acute esophageal necrosis is unclear, but appears to be multifactorial. According to the "two hit" hypothesis, there is an initial event (ie, low flow vascular state), which then predisposes the esophageal mucosa to a severe topical injury (ie, by reflux of acid and pepsin). Risk factors include vasculopathy, heart failure, hypovolemic shock, diabetic ketoacidosis, aortic dissection, alcohol intoxication, thromboembolic events, an underlying malignancy, peptic ulcus, gastric outlet obstruction, malnutrition, esophageal trauma and infections (ie, Candida albicans, cytomegalovirus, herpes virus, and Klebsiella pneumoniae) [6-14].
Over 90 percent of patients present with upper gastrointestinal bleeding including hematemesis, coffee-grounds emesis, melena and blood loss anemia. Other symptoms include dysphagia, epigastric pain, nausea, vomiting, fever and syncope [15, 16]. However, one report noted an asymptomatic black esophagus in a cancer patient [12]. Endoscopic findings are diagnostic, defined by a striking diffuse circumferential black appearance of the esophageal mucosa. Histologically a complete mucosal necrosis is seen sometimes extending to the muscular wall [15].
It is not necessary to obtain a tissue sample for diagnosis, except if we suspect an infectious origin where it may be necessary to perform virological or fungal analyzes (cytomegalo virus, herpes simplex virus, etc) especially in immunosuppressed patients. The initial treatment is conservative and consists of intravenous fluids and treatment of the underlying illness. Patients should be be kept nil per os (NPO) and treated with an intravenous proton pump inhibitor. In case of obstruction with vomiting the use of nasogastric tube should be done with extreme caution. Antibiotics should be considered on an individual patient basis.
Anemia should be corrected by red blood cell transfusion. In some cases it could be necessary an early surgical intervention when esophageal perforation is suspected [16]. Complications are rare, include strictures or stenoses in up to 10% of cases, appearing as early as 1 to 2 weeks after the diagnosis usually amenable to future endoscopic dilatation [3]. AEN related mortality is low (6%-12%), related to oesophageal perforation, mediastinitis and abscess formation. Overall mortality rate of nearly 32% is attributed to the severity of clinical conditions (older age, higher incidence of malignancy, chronic conditions [15, 16].
Case Report
A 52 years-old female, with past medical history of Wolf-Parkison-White syndrome and an uterine fibroid surgery had a diagnosis of Ewing sarcoma in April 2018. She had a pelvic mass and bone metastasis includind the second dorsal vertebra (D2) with spinal involvement and dorsal pain of strong intensity. She received external beam radiotherapy at the level of D2 for a total of 30 Gy, achieving a good pain control. Four days later he began chemotherapy with cyclophosphamide (1200 mg / m2) plus doxorubicin (37.5 mg / m2) and vincristine (2 mg).
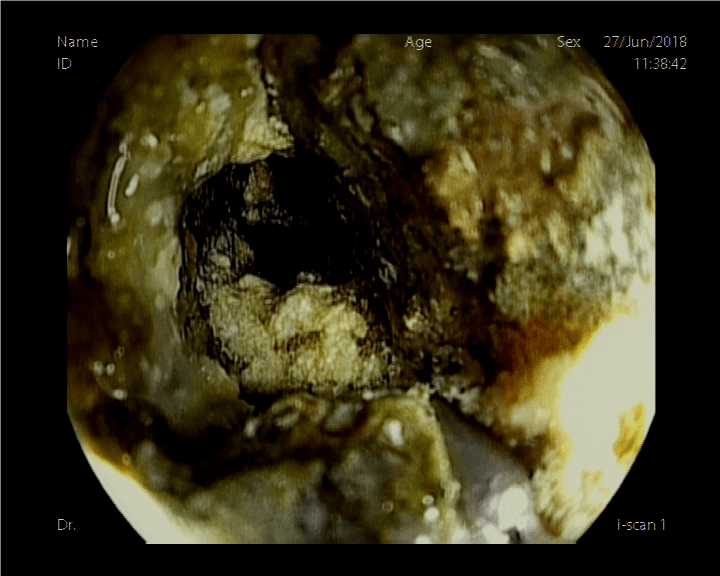
The patient had a good clinical response. After the first cycle of chemotherapy the patient suffered grade 3 hematological toxicity requiring blood transfusion. Four days after the second cycle of chemotherapy she started with epigastric pain and moderate dysphagia, so she received treatment with omeprazole and soft diet. After 2 weeks there was worsening with total dysphagia and the patient had an urgent upper endoscopy. The findings were complete obstruction 22 cm from the dental arch with a completely necrotic mucosa even with visualization of the esophageal muscle layer (Figure 1).
Figure 1: Diagnostic endoscopy.
The patient was hospitalized with the diagnosis of AEN. Physical examination revealed a good performance status, without fever, nor dehydration and with hemodynamic stability. We performed a body-TAC to ruling out complications (pneumothorax, perforation, etc.). Laboratory analysis showed a hemoglobin value of 10.5 g / dl, white blood cell count of 12,260 / mL, normal coagulation profile, albumin at 3.7 g / dL, and a creatinine value of 0.58 mg / dl. No liver function abnormality or hydroelectrolyte imbalance were found.
The treatment consisted of nil per os (NPO), high-dose protein pump inhibitor therapy (PPI) plus hyaluronic acid and chondroitin sulfate suspensions. We began parenteral nutrition as well as empirical intravenous antibiotics. The patient was discharged in a stable condition with parenteral home nutrition. Three weeks later she had an upper GI and small bowel series that showed normal passage of oral contrast through the esophagus with smooth mucosa and normal caliber except for a narrowing zone in upper thoracic esophagus (Figure 2).
Figure 2: GI and small bowel series.
On an upper endoscopy at 6 weeks there was improvement of the nectrotic changes and visualization of granular fibrin-covered areas with no stenosis (Figure 3). Then the patient started progressive oral diet without any incident.
Figure 3: Six weeks upper endoscopy.
In a subsequent body-TAC control there was progression of the Ewing sarcoma with impairment of the patient functional status. After a multi-disciplinary team discussion, supportive care is decided. She died on September 29, 2018.
Discussion
Acute esophageal necrosis is a rare disorder with low clinical incidence. The etiology is not clear although several theories have been proposed. An ischemic phenomenon seems to be the most frequently associated event ("two hit" hypothesis). There is evidence suggesting a temporary reduction in esophagel blood flow and decreased perfusion wich may lead to rapid development of extensive esophageal necrosis. It may be reversible when flow is restored [6]. In our patient there were two factors that can be associated with relative ischemia at esophageal level: external radiotherapy at second dorsal vertebra (D2) and anemization due to chemotherapy treatment. Furthermore, the thoracic esophagus received certain amount of ionizing radiation which implies a direct trauma at the level of the esophageal mucosa (two hit hypothesis).
Despite the low incidence, many cancer patients have risk factors for AEN. Five of the 23 patients reviewed by Lacy et al., had malignancies [4]. Although 90 percent of patients with AEN present with upper gastrointestinal bleeding, physicians should be alert of early symptoms such as those presented by this patient (epigastric pain and dysphagia). The diagnosis must not delay. Endoscopic findings are usually found at the level of the distal esophagus which is usually the area with less blood perfusion. Although proximal involvement has been described [17, 18]. In this case, the necrosis is described from 22 cm of the dental arch probably related to external radiotherapy at D2 level.
The management was conservative - according to practically all the case series consulted - with NPO, intravenous hydration and high doses of PPI. However, hyaluronic acid and chondroitin sulfate suspensions were used and it’s not a common approach. Parenteral nutrition, blood replacements and antibiotics were used prophylactically even without demonstrating over-added infection or complications such as perforation. The use of antibiotics is not standardized and the recommendation is to use them on an individual patient basis. The clinical response to treatment was satisfactory and allowed hospital discharge in days. The radiological control showed an improvement of the obstruction only at 3 weeks and the endoscopic control at 6 weeks showed a significant improvement of the appearance of the mucosa with healing areas.
Conclusion
Acute esophageal necrosis (AEN) is a rare medical disorder characterized by diffuse circumferential black esophageal mucosa. Diagnosis is based on esophagogastroduodenoscopy. The etiology of the disease remains unknown in most cases, though several factors are proposed to cause it such as ischemia and infections. We reported a patient with acute necrotizing esophagitis with an underlying malignancy treated with Chemotherapy and external radiotherapy. Both of them could have damaged the esophageal mucosa through ischemia and direct damage. Treatment consisted of NPO, intravenous fluids, high doses of PPI, hyaluronic acid and chondroitin sulfate suspensions, parenteral nutrition, and prophylactic antibiotics with satisfactory response. AEN must be recognized early and managed aggressively to improve clinical outcomes.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Mon 02, Mar 2020Accepted: Sat 28, Mar 2020
Published: Mon 30, Mar 2020
Copyright
© 2023 Zenzola Víctor. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.03.11
Author Info
Barranco Cao Raquel Garzón Carlos Herrera María Molina Mercedes Ortega Ruipérez Carolina Zenzola Víctor
Corresponding Author
Zenzola VíctorDepartment of Medical Oncology. Infanta Elena University Hospital. Valdemoro. Madrid, Spain
Figures & Tables


References
- Obermeyer R, Kasirajan K, Erzurum V, Chung D (1998) Necrotizing esophagitis presenting as a black esophagus. Surg Endosc 12:1430-1433. [Crossref]
- Geller A, Aguilar H, Burgart L, Gostout CJ (1995) The black esophagus. Am J Gastroenterol 90: 2210-2212. [Crossref]
- Gurvits GE, Cherian K, Shami MN, Korabathina R, El-Nader EM et al. (2015) Black esophagus: new insights and multicenter international experience in 2014. Dig Dis Sci 60: 444-453. [Crossref]
- Lacy BE, Toor A, Bensen SP, Rothstein RI, Maheshwari Y (1999) Acute esophageal necrosis: report of two cases and a review of the literature. Gastrointest Endosc 49: 527-532. [Crossref]
- Ben Soussan E, Savoye G, Hochain P, Hervé S, Antonietti M et al. (2002) Acute esophageal necrosis: a 1-year prospective study. Gastrointest Endosc 56: 213-217. [Crossref]
- Haviv YS, Reinus C, Zimmerman J (1996) "Black esophagus": a rare complication of shock. Am J Gastroenterol 91: 2432-2434. [Crossref]
- Cattan P, Cuillerier E, Cellier C, Carnot F, Landi B et al. (1999) Black esophagus associated with herpes esophagitis. Gastrointest Endosc 49: 105-107. [Crossref]
- De la Serna-Higuera C, Martinez J, Martin-Arribas MI, Rodriquez-Gomez S, Perez-Villoria A et al. (2001) Acute necrotizing esophagitis. Gastrointest Endosc 54: 225.
- Saftoiu A, Cazacu S, Kruse A, Georgescu C, Comanescu V et al. (2005) Acute esophageal necrosis associated with alcoholic hepatitis: is it black or is it white? Endoscopy 37: 268-271. [Crossref]
- Augusto F, Fernandes V, Cremers MI, Oliveira AP, Lobato C et al. (2004) Acute necrotizing esophagitis: a large retrospective case series. Endoscopy 36: 411-415. [Crossref]
- Katsinelos P, Christodoulou K, Pilpilidis I, Papagiannis A, Xiarchos P et al. (2001) Black esophagus: an unusual finding during routine endoscopy. Endoscopy 33: 904. [Crossref]
- Rejchrt S, Douda T, Kopacova M, Siroky M, Repak Rnet al. (2004) Acute esophageal necrosis (black esophagus): endoscopic and histopathologic appearance. Endoscopy 36: 1133. [Crossref]
- Rigolon R, Fossà I, Rodella L, Targher G (2016) Black esophagus syndrome associated with diabetic ketoacidosis. World J Clin Cases 4: 56-59. [Crossref]
- Choksi V, Dave K, Cantave R, Shaharyar S, Joseph J et al. (2017) "Black Esophagus" or Gurvits Syndrome: A Rare Complication of Diabetic Ketoacidosis. Case Rep Gastrointest Med 2017: 4815752. [Crossref]
- Abdullah HM, Ullah W, Abdallah M, Khan U, Hurairah A et al. (2019) Clinical presentations, management, and outcomes of acute esophageal necrosis: a systematic review.Expert Rev Gastroenterol Hepatol 13: 507-514. [Crossref]
- Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG (2007) Acute esophagea l necrosis:a rare syndrome J Gastroenterol 42: 29-38. [Crossref]
- Gurvits GE, Robilotti JG (2010) Isolated proximal black esophagus: etiology and the role of tissue biopsy. Gastrointest Endosc 71: 658. [Crossref]
- Neumann DA 2nd, Francis DL, Baron TH (2009) Proximal black esophagus: a case report and review of the literature. Gastrointest Endosc 70: 180-181. [Crossref]
