A Unique Case of Chronic Myeloid Leukemia Occurring in an Adolescent Male
A B S T R A C T
Background: Patients with chronic myeloid leukemia (CML) usually present in elderly age and rarely in the adolescents and young adults (AYA) population. There is a limited understanding of disease management and survival outcomes in this group of patients.
Case Presentation: An 18-year-old man presents to our hospital for abdominal pain and weight loss. Examination pertinent for splenomegaly and labs significant for profoundly elevated white cells count. FISH was positive for BCR/ABL1 fusion gene, and thus, he was diagnosed with CML and treated with TKI with clinical improvement.
Conclusion: Tailoring CML novel therapeutic strategies in the AYA population to different disease characteristics will better advance our understanding of this disease in this patient population.
Keywords
Chronic myeloid leukemia, adolescents and young adultstyrosine, kinase inhibitor
Background
Chronic myeloid leukemia (CML) is a chronic myeloproliferative neoplasm that has been reported that it commonly presents in the elderly with a median age of 67 years [1]. CML is uncommonly in adolescents and young adults (AYA) between the age of 15-29 years [2]. The biology of the condition, outcome, and response to therapy remains uncertain in this age group [3]. In this report, we describe a case of an 18-year-old man who presents to our hospital with abdominal pain found to have extensive splenomegaly with leukocytosis; was diagnosed with CML.
Case Report
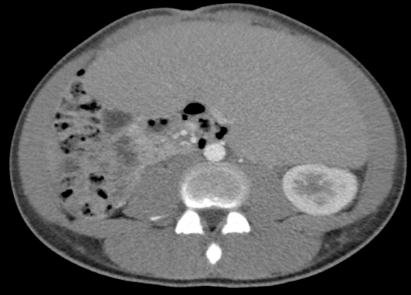
An 18-year-old Hispanic man with no prior medical history presents to our hospital complaining of abdominal pain and 12-pound weight loss over one month before presentation. Upon physical examination, the patient was found to have extensive splenomegaly. His laboratory workup was pertinent for hemoglobin of 7.3 g/dL, platelets count of 235×109/L. His white blood cells count of 622 ×109/µL with 14%neutrophils, 30% bands, 11% metamyelocytes, 30% myelocytes, 7% promyelocytes, 1% blasts, 2% lymphocytes, and 5% eosinophils. The peripheral smear revealed a range of granulocytic cells with less than 3% blasts (Figure 1). Imaging comprised of computed tomography (CT) scan of the abdomen confirmed extensive splenomegaly (Figure 2). Fluorescent in situ hybridization (FISH) was positive for BCR/ABL1 fusion genes. Bone marrow aspirate and core biopsy specimen revealed morphologic findings consistent with CML, including marked hypercellularity, myeloid hyperplasia, and prominent granulocytic lineage without evidence of increased blasts (Figures 3 & 4).
Flow cytometry showed atypical myeloid findings, left shift, increased CD57, and 7% blasts. Therefore, the patient underwent leukapheresis and started on hydroxyurea, allopurinol, and dasatinib, a tyrosine kinase inhibitor (TKI). During his admission, he became increasingly hypoxic, for which a high-resolution CT (HRCT) scan of his chest with contrast revealed bilateral lower lobe consolidation. Therefore, the patient was started on an empiric antibiotic therapy and later discontinued after all infectious workup, including blood and respiratory cultures, remained negative. Hypoxemia considered most likely due to pulmonary leukocytic infiltrates. The patient’s clinical status then improved, and he was subsequently discharged from the hospital with a follow-up with the hematology clinic in the outpatient setting.
Figure 1: Peripheral Smear at 400x magnification demonstrating a profound leukocytosis with a range of myeloid cells from rare blasts to mature segmented neutrophils.
Figure 2: CT abdomen showing splenomegaly of 31 centimeters.
Figure 3: Bone marrow aspirate smear at 400x magnification: Cellularity is markedly increased. The myeloid to erythroid ratio is approximately 20:1 with marked myeloid hyperplasia. Granulocytic cells demonstrate maturation to segmented forms without evidence of increased blasts. Blasts account for approximately 1% of the marrow cellularity.
Figure 4: Bone marrow core biopsy at 400x magnification: The marrow is 100% cellular. The granulocytic lineage is predominant as in the aspirate smear. There is obvious maturation to segmented forms without identifiable increased blasts.
Discussion
CML has historically been identified as a disease of the elderly population. However, current literature reports that adolescents and young adults are also susceptible, with inadequate knowledge of the disease survival outcomes in this specific patient population. CML is usually distinguished by t(9;22) (q34;q11.2) genetic translocation leading to fusion of Abelson murine leukemia (ABL1) gene with breakpoint cluster region (BCR) gene that leads to an ungovernable activity of the tyrosine kinase BCR-ABL1 fusion protein. This genetic translocation making it a target for novel TKI therapy [4-6]. The course of this disease results in the deregulation of hematopoiesis, resulting in a wealth of immature granulocytes [7]. Splenomegaly is one of the most prevalent findings during physical examination in up to half of CML patients.
This, together with anemia, results in including fatigue, weight loss, and abdominal pain, as seen in our patient. AYA population have often presented differently compared with other age population, they more often with a higher WBC and higher incidence of a larger spleen size opposed to other age groups. Various other clinical presentations could have been seen. Our patient developed an acute pulmonary complication which believed to be secondary to pulmonary lymphocytic infiltration given negative infectious workup [8, 9]. Up to half of the CML patients in the United States are asymptomatic at the time of diagnosis.
CML described having a triphasic nature with the chronic phase being the most common CML phase, which is reported to be present in up to 90% of cases with a potential progression into accelerated and blastic phases. Therefore, it exhibits a high variability in clinical prognosis [7]. This prognostic variation remains in many ways unclear for AYA patients [10]. Furthermore, current prognostic scoring systems recognize age as an essential risk factor and fail to take into account this subset of patients [9, 11, 12]. Provided that our patient was in the chronic disease phase, the outcome suggested to be more favorable when compared to accelerated or blast phase presentation CML [4]. Multiple factors play a role in TKI treatment decision. Most TKI therapies are usually well-tolerated.
Patients with comorbidities or patients at risk of developing certain medical conditions play a role in therapy decisions. Moreover, allogeneic stem cell transplantation is not recommended as initial treatment for patients with chronic phase CML. Novel second-generation TKI therapy indicated because of the achieved enhanced survival advantages. Second-generation TKI therapy is considered in younger patients with CML because of the increased rates of complete molecular responses in this patient population compared to older patients and thus eventually leading to molecular cure and, ultimately, therapy discontinuation [8]. However, the AYA subset of patients appears to have a lower response to TKI therapy when compared to older adults. The various biological and metabolic characteristics, poor treatment adherence, and difficult dose adjustment in the AYA subset of patients may have contributed to different disease outcomes [9].
Conclusion
There is paucity in the understanding of the prognostic features, management, and outcomes for CML in AYA patients. The knowledge of these predictive factors may help better understand the clinical characteristics of this patient population. This report highlights the necessity for further research focusing on individualized novel treatment strategies to be tailored to various distinctive features of this disease to achieve a better understanding of disease outcomes.
Article Info
Article Type
Case ReportPublication history
Received: Mon 02, Mar 2020Accepted: Fri 27, Mar 2020
Published: Tue 31, Mar 2020
Copyright
© 2023 Dawood Findakly. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.03.13
Author Info
Dawood Findakly Mohammed Al-Charakh Surabhi Amar
Corresponding Author
Dawood FindaklyCreighton University School of Medicine, Omaha, Nebraska, USA
Figures & Tables



References
- Oran B, Weisdorf DJ (2012) Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica 97: 1916-1924. [Crossref]
- Aben KK, Gaal CV, Gils NA, van der Graaf WT, Zielhuis GA (2012) Cancer in adolescents and young adults (15-29 years): A population-based study in the Netherlands 1989-2009. Acta Oncol 51: 922-933. [Crossref]
- Andolina JR, Neudorf SM, Corey SJ (2012) How I treat childhood CML. Blood 119: 1821-1830. [Crossref]
- Thompson PA, Kantarjian HM, Cortes JE (2015) Diagnosis and Treatment of Chronic Myeloid Leukemia in 2015. Mayo Clin Proc 90: 1440-1454. [Crossref]
- Siu LL, Ma ES, Wong WS, Chan MH, Wong KF (2009) Application of tri-colour, dual fusion fluorescence in situ hybridization (fish) system for the characterization of bcr-abl1 fusion in chronic myelogenous leukaemia (cml) and residual disease monitoring. BMC Blood Disord 9: 4. [Crossref]
- Shtivelman E, Lifshitz B, Gale RP, Canaani E (1985) Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature 315: 550-554. [Crossref]
- Che YQ, Shen D, Zhang SM, Qi J (2014) Identification of immature granulocytes in cancer chemotherapy patients by cell counting vs. microscopic examination of blood smears. Mol Clini Oncol 2: 207-211. [Crossref]
- Jabbour E, Kantarjian H (2018) Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol 93: 442-459. [Crossref]
- Castagnetti F, Gugliotta G, Baccarani M, Breccia M, Specchia G et al. (2015) Differences among young adults, adults and elderly chronic myeloid leukemia patients. Ann Oncol 26: 185-192. [Crossref]
- Kantarjian H, O’Brien S, Keating M, Beran M, Estey E et al. (1997) Results of decitabine therapy in the accelerated and blastic phases of chronic myelogenous leukemia. Leukemia 11: 1617-1620. [Crossref]
- Tricoli JV, Seibel NL, Blair DG, Albritton K, Hayes Lattin B (2011) Unique characteristics of adolescent and young adult acute lymphoblastic leukemia, breast cancer, and colon cancer. J Natl Cancer Inst 103: 628-635. [Crossref]
- Pemmaraju N, Kantarjian H, Shan J, Jabbour E, Quintas Cardama A et al. (2012) Analysis of outcomes in adolescents and young adults with chronic myelogenous leukemia treated with upfront tyrosine kinase inhibitor therapy. Haematologica 97: 1029-1035. [Crossref]
