A Review of Pre-Clinical Studies for the Treatment of Neonatal Brain Injury
A B S T R A C T
The developing brain is especially sensitive to perturbations such as hypoxia-ischemia (HI) or surgical ablation in the perinatal period. We first review and contrast the effects perinatal HI and surgical perturbation in laboratory rats. The developing brain is also very responsive to a wide range of other experiences that can induce remarkable neural plasticity in both the normal and perinatally injured brain. We next review the factors that influence this plasticity in both the normal and perinatal injured. We consider treatments that stimulate cerebral and behavioural plasticity, especially in the motor systems. The goal is to draw attention to possible treatments that could be translated from perinatal surgical ablation to the HI model and eventually to the clinic.
Keywords
Perinatal injury, post-injury therapy, neural plasticity, FGF-2
Introduction
The incidence of brain injury in babies is relatively high ranging from about 3 per 1000 in full term infants to about 25 per 1000 in preterm births (babies born at or less than 37 weeks) [1, 2]. The only effective treatment that is standard practice is hypothermia, but preclinical studies in laboratory animals have examined a wide range of other options ranging from neuroprotective agents to behavioral therapies. The neuroprotective therapies have been reviewed in detail recently so our focus will be on other types of therapies that have been shown to positively influence functional recovery [2]. Here we briefly review the stages of brain development and neural plasticity before examining models of perinatal brain injury and the major factors that modulate brain development after perinatal surgical ablation and HI brain injury.
Although there are large literatures on both surgical ablation and HI models, these literatures have very different emphases. The surgical ablation studies have focussed on studying cerebral and behavioral plasticity during development whereas the HI studies have focussed more on mechanisms of injury, especially related to cerebral palsy, and possible clinical applications. One goal of this review is to demonstrate that these two solitudes have much to offer each other and should be seen as complementary. Because the HI model studies have often been focussed more on cerebral palsy, which is associated with significant motor disturbances, our emphasis is on examination of motor system plasticity in both the surgical ablation and HI studies.
Periods of Development
We can conceive of development as broadly falling into three stages, each of which can influence recovery from perinatal injury. The first, preconception, reflects how experiences can modify brain development by the (re)programming of later gene activity in the oocyte (in dams) and spermatocytes (in males). In other words, experiences can influence the expression of genes [3, 4]. Given that the male sperm cells turn over about every 60-90 days, experiences during this period can significantly alter the pattern of chromatin and histone formation, leading to changes in gene expression, and thus differences in the brains of animals with varying preconception experiences [5].
The second stage in placental mammals is the sequence of events in utero in which there is a genetically determined sequence of events that can be modulated by the maternal environment. This environment can be affected by stress, diet, drugs, etc. experienced directly by the mother, but can indirectly influence the developing infant through the placental blood. In utero brain development begins with the generation of neurons, which in rats is on embryonic (E) day 10.5-11 and in humans is around E28 making the nervous system one of the earliest human body systems to begin growing but the last to be completed. Neurogenesis is largely complete by birth (E21) in rats and about 5 months gestation in humans, although the rate and duration of neurogenesis varies with brain region (see https://embryology.med.unsw.edu.au).
As neurons are formed, they begin their migration to their final destination. The traditional view is that in rats and mice this continues into the postnatal period for about a week but is largely complete in human cerebral cortex by birth. There is some debate, however, over whether these conclusions are accurate. Mortera & Herculano-Houzel used a different method of cell counting (the istropic fractionator) to determine total numbers of neuronal and non-neuronal cells in the rat, and found that there is an increase in neuron number between 1-2 mo of age, which corresponds to the period of adolescence [6]. It is not yet known if a similar phenomenon occurs in humans, but if so, it would be an important time to target treatments to enhance neurogenesis that is already in progress [7].
The third stage is postnatal at a time that the brain is actively forming connections. Neural migration is mostly complete by about P7 in rats (P1 month in humans); the most intense dendritic growth is around P14 in rats (P8 months in humans); and, maximum synaptic density is reached about P35 in rats but in humans it ranges from about 2-5 years depending upon the location [8-10]. At the peak of synaptogenesis, the cerebral synapses are over-produced to about two times the adult number and in the prefrontal cortex are pruned during adolescence [9].
During the postnatal period the brain is strongly influenced by the ex utero environment, which includes all manner of experiences ranging from maternal-offspring interactions to sensory inputs (light, sound, touch, etc.), diet, stress, and so on. As in the second stage, these experiences can act to regulate gene expression leading to altered developmental outcomes [5]. This stage is prolonged, continuing well into the third decade of life in humans [11].
General Types of Brain Plasticity in Development
Brain plasticity can be shown using many levels of analysis ranging from behavior to molecules. Studies of human participants are largely restricted to studies of behaviour and functional organization using noninvasive imaging [12]. Studies of laboratory animals can include behaviour and noninvasive imaging but have the advantage of also being able to use more invasive imaging and to examine neuronal morphology, molecular structure, and epigenetics [13]. There is no correct level of analysis, the choice being suited to the questions asked and technology available. In our studies, we have chosen to use a combination of behaviour, neurophysiologically-defined motor maps, dendritic/synaptic morphology, neuronal generation, and epigenetics.
Three special features of developmental plasticity are especially important. The first feature is found in the cells lining the subventricular zone (SVZ) of the lateral ventricles and cells in the hilus of the dentate gyrus. Both regions contain stem cells that remain active throughout life. The cells in the SVZ produce both glial and neural progenitor cells that can migrate into cerebral gray or white matter, even in adulthood. The SVZ cells in rodents appear mostly quiescent but can become activated, largely in response to cerebral perturbations. For example, after perinatal injury these cells can produce neurons either spontaneously or in response to growth factors such as Fibroblast Growth Factor-2 (FGF-2) [14, 15]. In rodents, the SVZ stem cells also generate a continual stream of neural progenitors that travel to the olfactory bulb. Stem cells in the dentate gyrus generate new neurons at a slow but steady pace throughout life in both rodents and humans, although there is a decline with aging [16]. An important aspect of stem cell activity in the young brain is that it is possible to stimulate neurogenesis after an injury to facilitate recovery.
A second special feature of developmental plasticity is the speed at which dendrites, and especially dendritic spines, can modify their structure to form or delete synapses in response to experience, possibly in a matter minutes [17]. Modern neuroimaging technology such as two-photon imaging has allowed researchers to observe changes in spines as they occur in laboratory rodents [18]. Furthermore, it has recently become possible to use injections of radioligands with advance Positron Emission Tomography to imagine in vivo synaptic density in humans, although this has not yet been applied to the developing brain. Remodeling of dendritic fields is a key aspect of cerebral plasticity following early injury.
A final special feature is the presence of critical periods, especially for experience-expectant plasticity. One of the best studied phenomena was first described by Wiesel & Hubel who showed that if one eye of a kitten is kept closed after birth, the open eye expands its territory at the expense of the closed eye [19]. When the closed eye is eventually opened after a few months, its vision is compromised, resulting in an enduring loss of spatial vision (amblyopia) [20]. Recent work has shown that the critical period results from an appropriate balance of excitatory and inhibitory inputs (E/I). The maturation of inhibitory GABA circuits underlies the timing of onset of the critical periods, which vary across brain regions. Premature onset of the critical period is prevented by various factors including polysialic acid acting on neural cell adhesion molecules, which act on parvalbumin (PV) in GABA interneurons.
As other factors promote PV cell maturation the critical period begins. The critical period closes as molecular brakes emerge to dampen plasticity, alter the and thus limit adult plasticity [21]. A key point is that it is possible to reopen the critical period by manipulating the E/I balance chemically, providing a novel mechanism to stimulate recovery after perinatal injury. Although critical periods have historically been thought of as occurring early in postnatal development, there is growing evidence that adolescence may also be a critical period of neuronal plasticity [22]. Recall that Mortera & Herculano-Houzel [6] found evidence of cortical neurogenesis in puberty and maximum synaptic pruning in the prefrontal cortex also occurs during puberty [10]. Although we are not aware of lab studies that have delayed treatment for perinatal injury until puberty, it is possible that this may be an important therapeutic target time.
Models of Perinatal Brain Injury
In humans, perinatal brain injury can occur in both term and preterm infants. For term infants, the most common causes are hypoxia-ischemia or stroke, both of which lead to either focal or diffuse the death of neurons and glia. Cerebral injury in preterm infants is most often caused by chronic hypoxia resulting from immature lung development, but may also be related to infection/inflammation, which can continue postnatally, and intrauterine growth restriction [23, 24]. An increasingly common view of the complex nature of these types of perinatal injury involves antenatal factors (e.g., inflammation, maternal stress, genetic factors) that sensitize the brain making it more susceptible to a second perinatal insult such as HI or inflammation [25]. The postnatal sequelae of HI injuries are complex including the overproduction of glutamate, which is toxic, as well as increased pro-inflammatory cytokines, oxidative stress, disruption of glial maturation, and alteration of the extracellular matrix and perturbed maturation of neurons and glia [24, 26, 27]. The effect of these complications is that injuries are seldom focal but may be somewhat diffuse.
Animal models of HI perinatal injury most commonly use rats or mice [24]. Examples include either rearing animals with low levels of oxygen between postnatal (P) days 3 and 11 (a preterm model), or hypoxic-ischemic injury in which carotid artery occlusion is followed by hypoxia, typically from P3-P9 [28]. Other studies have combined HI and inflammation using a single dose of lipopolysaccharide (LPS) at P7 before unilateral occlusion as above [29].
Although the ideal model for perinatal hypoxia-ischemia (HI) in human infants might be the HI rodent model, there are other models that provide some advantages. The earliest systematic laboratory studies of early brain injury are those of Margaret Kennard who studied the effect of motor cortex lesions in infant monkeys [30, 31]. She compared the effect of unilateral motor cortex lesions in infant and adult monkeys finding milder impairments in the infants [3]. In these studies, anesthetized animals had the skull opened and tissue removed using gentle subpial aspiration, which is radically different than the effects of HI discussed earlier. Although Kennard did not study the postinjury changes in the brain of her monkeys, she did speculate on possible effects of the injuries on synaptic organization. It was Kennard’s studies that led to the idea that postnatal cortical injury early in development is associated with a better outcome than surgery later, an idea often referred to as the “Kennard Principle.”
By the 1970s an extensive literature began to accumulate with studies in monkeys, cats, ferrets, and various rodents (rats, mice, hamsters) receiving similar cortical ablation surgeries. The advantage of these studies is that the injuries can be directed to specific cortical regions, such as prefrontal or visual cortex, which is not practical with the HI lesion because there is no direct cerebral injury [32, 33]. HI injuries are quite variable (see Figure 1) with a general finding roughly half of the HI animals have infarctions [24]. More recent studies have used neurotoxins to produce focal injury, but these neurotoxins are difficult to use in the developing brain and do not work quite the same as in adulthood. One disadvantage of the ablation model is that the skull is opened, which is rare in children. Another disadvantage is that in contrast to the HI models there has been surprisingly little study of the postinjury sequela of the surgical interventions other than an examination of glial and neuronal death and genesis and changes in cerebral connectivity [33]. It is likely, however, that many of the post HI sequelae will also be occurring in the surgically perturbed brain.
The studies of HI/inflammation and surgical ablation are clearly aimed at answering different questions, but the two types of studies should be complementary. After all, they are both about the postinjury effects of perinatal injury and both models have studies looking at remedial treatments (see below). There are very few direct comparisons of the two types of etiologies, however. Our own studies began with surgical ablations at a variety of ages and places in the cerebral cortex and various treatments were used to stimulate recovery (or not) [34]. Based on these latter studies we shifted to the Vannucci model and used some of the same treatments as in our ablation studies as a proof of principle that the type of injury was much less important than the type of intervention [28].
One challenge in doing pre-clinical studies is in determining appropriate ages to compare lab animals and humans. For the ablation studies this is not usually an issue as the research question is really about how the brain responds to early injury at different developmental times but for the HI studies the question is more directly aimed at clinical issues. P7 is the most commonly used age for HI studies and the implication is that it is roughly equivalent to term infants. But for the purposes of comparison across the surgical ablation and HI studies we also consider other ages.
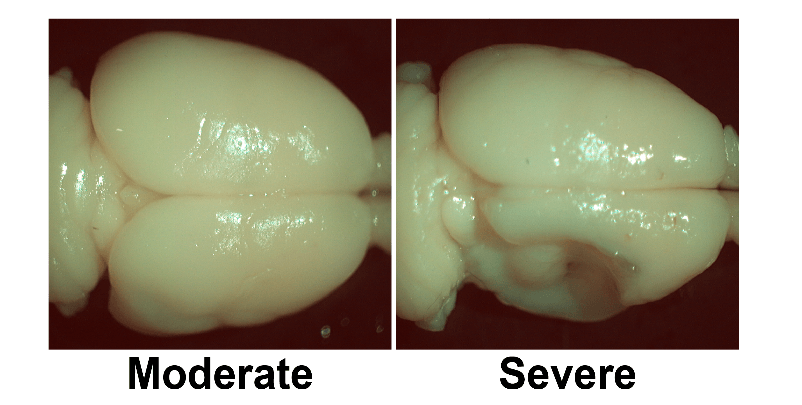
Figure 1: A comparison of two brains with P7 HI events in which both animals received permanent coagulation of the right common carotid artery and exposure to hypoxia (8% O2 for 1.5h). The right hemisphere is shrunken in both cases but there is obvious infarction in the severe case [35].
The Effects of Perinatal Ablations
As noted above, the first systematic ablation studies were those of Margaret Kennard who made unilateral motor cortex lesions in infant and adult monkeys and found milder impairments in her younger animals. Kennard was aware that her monkeys still had motor deficits but her studies led to the idea that “earlier is better” [36]. In contrast, in his studies of children with perinatal frontal lobe injuries Hebb concluded that “earlier is worse” because the frontal injury was interfering with developing neural networks [37-39]. Subsequent laboratory studies over the past 40 years have shown that the outcomes depend up the precise age at injury, age at assessment, whether injury the injury is bilateral or unilateral, and the behavioral measurements used. A key consideration is not the age post birth in different species but rather the post conception developmental age. Rats and mice are born sooner post conception, and thus less developed, than cats, for example, and monkeys are born more mature than cats or humans.
Examination of the effect of early cortical injuries in rats has compared the effects of lesions at different ages on a wide range behavioral measures including cognitive, motor, and species typical tests [33]. In general, the results across studies using rodents show that bilateral focal damage to presumptive medial prefrontal cortex (mPFC) during the latter part of neurogenesis (E18 in rats) allows good outcomes even though the brain is strikingly abnormal [40]. For example, there are gross abnormalities in frontal cortical architecture including both gray and white matter. This abnormal morphology is particularly striking given that the functional outcomes in these animals is essentially indistinguishable from normal control animals. Damage to the mPFC during neural migration and early synapse formation (P1-6 in rats) leads to severe behavioral and neural abnormalities: the brains are very small, the entire cortical mantle is thin, and there is marked dendritic stunting [41-43]. In contrast, damage during dendritic and synaptic expansion (P7-12 in rats) leads to a much better functional outcome, which is correlated with dendritic hypertrophy and spontaneous neurogenesis [14, 43, 44]. We note here, that although lesions at P7-12 allow good recovery of cognitive behaviors, there is less recovery of motor behaviors and no recovery of species typical behaviors [45].
Similar patterns of age-related functional outcomes can be seen in cats and monkeys although the ages vary because of differences in gestational rate [46, 47]. The age-related differences in outcome is important for studies of the effects of treatments, in part because animals with mild deficits show less benefit than animals with more severe impairments.
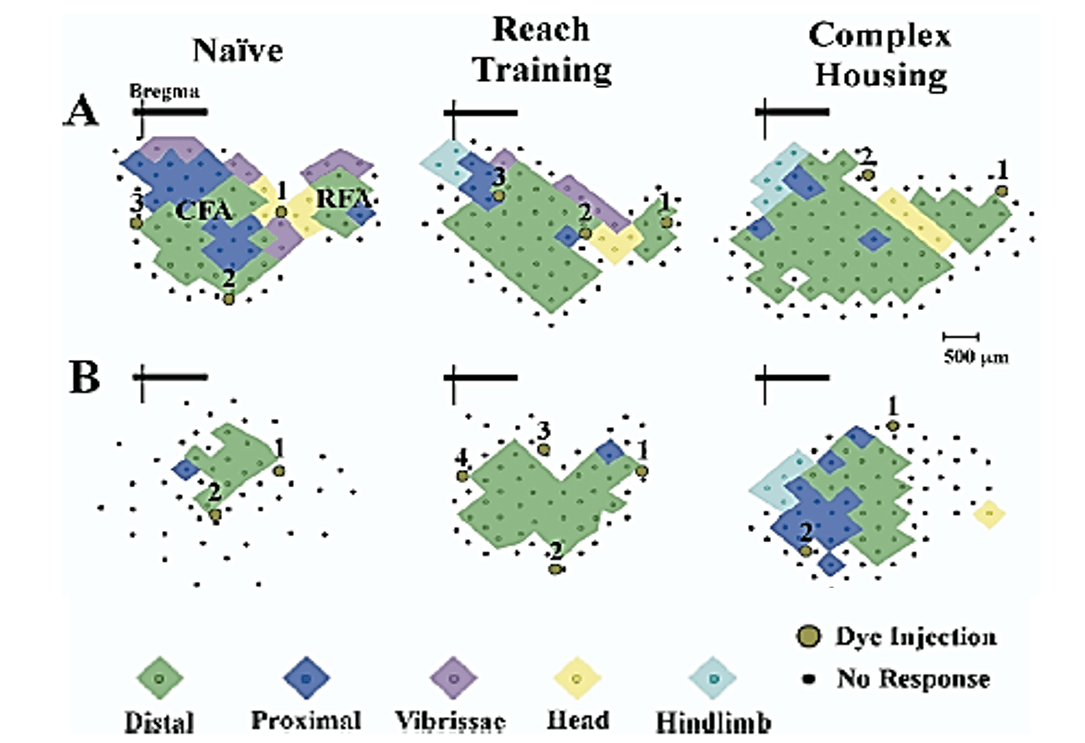
In contrast to the effects of bilateral focal lesions, large unilateral lesions (e.g., hemidecortication) show a different pattern of results in which the earliest injuries (P1-P3) show better outcomes than later injuries (P5, P10) [48, 49]. This difference likely reflects the role of the undamaged hemisphere in the compensatory mechanisms underlying functional recovery. Thus, for example, in contrast to rats with adult hemidecortications, rats with hemidecortications in the first few days of life have increased cortical thickness in the remaining cortex, even though there is no corpus callosum. The compensatory increase in cortical thickness is due, in part, to general hypertrophy of cortical pyramidal neurons [49]. In addition, there is extensive anomalous wiring from the intact hemisphere to contralateral subcortical regions. These changes are much attenuated in rats with P10 hemidecortications [49]. Curiously, rats with unilateral medial prefrontal lesions have shrunken motor maps on the ipsilateral side but relatively normal maps on the intact side (Figure 2) [50]. By using intracortical microstimulation (ICMS) it is possible to identify two forelimb control regions, a larger one referred to as the caudal forelimb area (CFA), and a smaller one called the rostral forelimb area (RFA), which is located in front of the head area, as shown in (Figure 2). Medial prefrontal cortex lesions at P10 reduced the size of the ipsilateral CFA and totally removed the RFA, even though the maps were quite distal from the lesion site.
Figure 2: Forelimb motor maps. Computer-generated graphic showing forelimb motor maps derived from intracortical microstimulation in control (A) and unilateral medial frontal cortex suction lesion. (B) Left to right shows the organization of forelimb representations in naïve, reach trained, and complex housed animals. The lesion brain has markedly smaller forelimb representation with a markedly smaller caudal forelimb area (CFA) and no rostral forelimb area (RFA). Treatments increased the size of the maps in both intact and lesion animals although the effect was larger in the intact animals. Brown circles indicate location of Toludine Blue injections (1,2,3,4) used to mark the extent of the motor maps. Note that there is no shift in motor map position relative to the bregma [50].
The Effects of Perinatal HI
It is more difficult to identify age-related differences for the HI injuries because most studies that examine behavior after HI have focused on injury at P7. Overall, studies have shown a range of behavioral phenotypes including motor deficits, impaired spatial memory and learning, deficits in sensory processing, and reduced attention [51, 52]. In an attempt to compare the effects of ablation and HI results Williams did a series of studies comparing the functional effects of HI at P3, P7, and P14 on motor behavior and the size of cortical motor maps [53]. By using ICMS in anesthetized rats as in the earlier ablation studies (Figure 2) Williams identified regions of forelimb control.
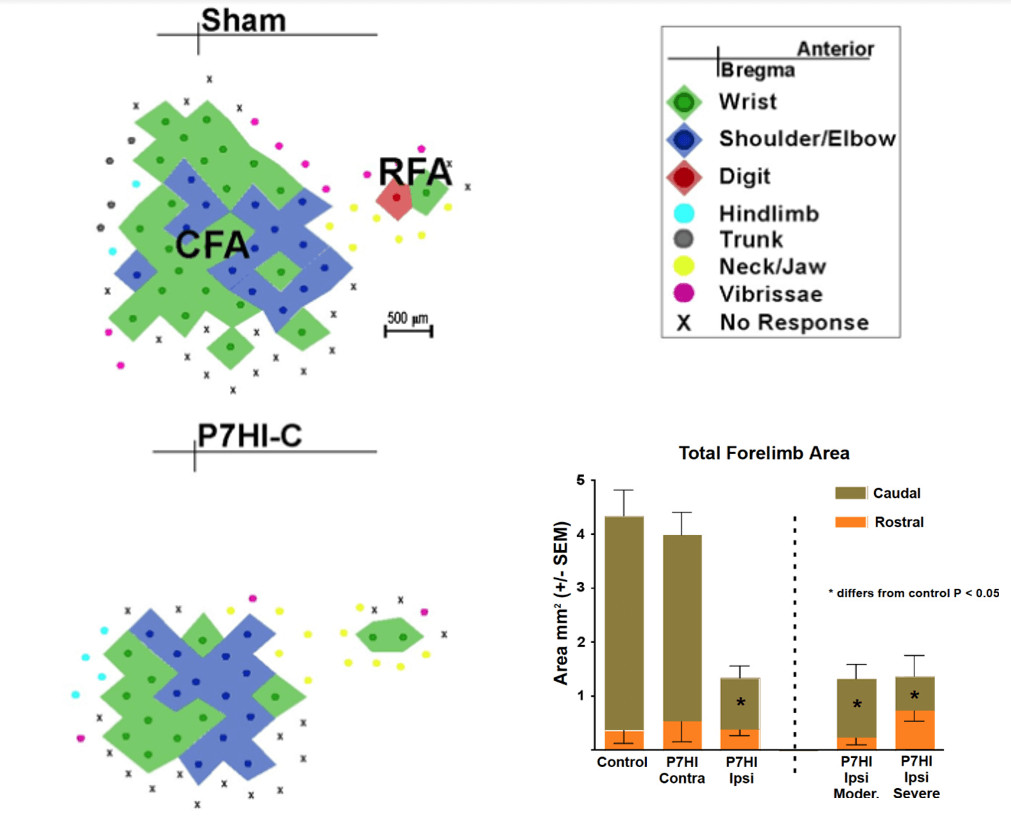
The results showed that HI on P7 or P14 impaired skilled reaching performance, which was correlated with a reduction in the size of the caudal motor representation in adulthood, much as in the ablation study [53]. In contrast, HI at P3 resulted in increased size of motor maps on the injured hemisphere and better motor performance than seen in the animals with later injuries [54]. Figure 3 illustrates effect of P7 HI on the motor map relative to a sham map and shows that only the ipsilateral hemisphere is affected and that the lesion size illustrated in (Figure 1) had no effect on the motor map size. Notice that in contrast to the effect of mPFC ablations, the P7HI lesions did not abolish the RFA, even in the severely damaged brains.
Figure 3: Reconstructed motor maps representing the forelimb derived from intracortical microstimulation in rats with P7 HI injuries. Shown are a typical caudal forelimb area (CFA) and rostral forelimb area (RFA) (Sham, top left), and representative map from P7 HI. The CFA is dramatically reduced in size in the HI brain [54].
The Basis of Treatment Strategies for Perinatal Injuries
The place to begin with looking for treatment strategies for cerebral injury is to consider the factors positively affecting the synaptic organization and function of the normal brain (Table 1). It is clear from the table that both behavioural and pharmacological experiences can influence brain plasticity and behaviour in the normal brain. We hasten to point out that many other factors can also influence brain and behaviour, but in a negative manner, the best examples being stress and psychoactive drugs [55-57].
Table 1: Factors positively affecting the synaptic organization and enhanced behavioural performance in otherwise normal animals.
|
Factor |
Example references |
|
[58] |
|
[35] |
|
[60] |
|
[40] |
|
[61] |
|
[62] |
|
[63] |
|
[64] |
|
[65] |
|
[66] |
|
[67] |
|
[68] |
Applications to The Perinatally Injured Brain
Although many of the treatments used for animals with adult lesions have been applied to those with perinatal injuries, the bulk of the studies have been on animals with ablations, but there is little reason to believe that they will not also be effective in animals with HI injuries. One difference in the use of treatments in young versus adult animals is that infant animals have a restricted range of behaviors so many behavioral therapies cannot be started as quickly in the young animals. But pharmacotherapies can be started immediately and, in some cases, gestationally. The latter effect is fascinating because the treatment can be given before the injury occurs, as might be desirable in clinical populations with women at risk for birth complications.
The first type of treatment to be used to stimulate recovery after perinatal injury was complex housing. Bland & Cooper first showed that housing rats in complex environments after perinatal ablation of the visual cortex significantly improved functional outcome and this finding was later replicated in kittens with occipital ablations [69, 70]. This general effect has been replicated numerous times in animals with perinatal injuries in other cortical regions such as motor or medial prefrontal cortex. Indeed, in general, housing animals in complex environments that allow social interaction, novelty (objects to interact with that are changed regularly) and unlimited exercise is one of the most powerful treatments for animals with perinatal brain injury, even if it is not begun until adulthood.
For example, rats with perinatal mPFC ablations have impairments in fine motor tasks such as skilled reaching and grasping and this is correlated with abnormal organization of forelimb motor maps, even if the motor cortex itself is not injured [50]. Placing similar animals in complex environments in adulthood improves skilled motor function, which is correlated with reorganization of the motor maps whereby the size of the forelimb motor maps increases to roughly normal size (Figure 2). There are also many studies showing that complex housing also improves both motor and cognitive outcomes and increases spine density after HI at P7, which nicely parallels the effect on rats with perinatal ablations [71, 72].
Table 2: Factors improving behavior after surgical ablation or HI perinatal injury.
|
Factor |
Model |
Example References |
Outcome |
|
Behavioral therapies |
|||
|
SA |
[70] |
é vision |
|
SA |
[73] |
é vision |
|
HI |
[72] |
écognition, émotor |
|
SA |
[74] |
émotor |
|
SA |
[59] |
écognition, émotor |
|
HI |
[52] |
écognition, émotor |
|
SA |
[75] |
émotor |
|
HI |
[76] |
écognition, émotor |
|
Pharmacotherapies |
|||
|
SA |
[77] |
é vision |
|
SA |
[66] |
écognition, émotor |
|
|
SA |
[77] |
émotor |
|
HI |
[53] |
écognition, émotor |
|
HI |
[78] |
écognition, émotor |
|
SA |
[79] |
émotor |
|
HI |
[80] |
écognition, émotor |
|
HI |
[81] |
émotor |
|
SA |
[82] |
écognition |
|
HI |
[83] |
écognition |
écognition, émotor indicates improved performance on cognitive or motor tasks.
SA: surgical ablation; HI: hypoxic/ischemic. Treatments are given postinjury unless noted otherwise.
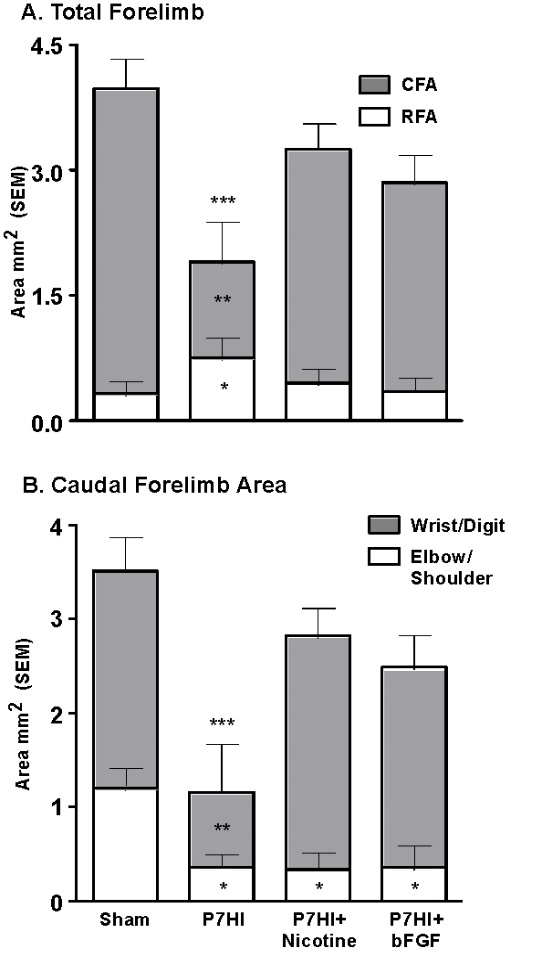
Another very strong modulator of perinatal injury is tactile stimulation (TS). Gentle stroking with a brush for 15 min, three times per day, for 2 weeks after P4 mPFC or parietal cortex injury dramatically improves the behavioural outcomes in adulthood [74]. One mechanism is an increase in Fibroblast Factor-2 (FGF-2) production in the skin. FGF-2 passes the blood brain barrier and stimulates receptors in the brain, which in turn leads to increased dendritic arborization and spine density in cortical pyramidal neurons. Furthermore, tactilely stimulating the pregnant females has similar behavioural and anatomical effects on the offspring who subsequently have mPFC lesions on P4 [59]. Subcutaneous postinjury administration of FGF-2 for 3 days after perinatal injury or to the pregnant dams prior to perinatal injury also stimulates improved function (Figure 4). Both treatments were effective in dramatically reducing the behavioural effects of P4 mPFC or parietal cortex ablation lesions [77]. Post HI administration of FGF-2 or nicotine also stimulates motor recovery and increases the size of the caudal motor region. The increased size is largely due to an increase in representation of the wrist and digits, which would mediate the improved skilled reaching. In hindsight, it is unfortunate that the authors did not examine for possible neurogenesis.
Figure 4: Treatment with either FGF-2 or nicotine post P7 HI increases the size of the forelimb representation relative to untreated animals and this is correlated with enhanced motor performance [53].
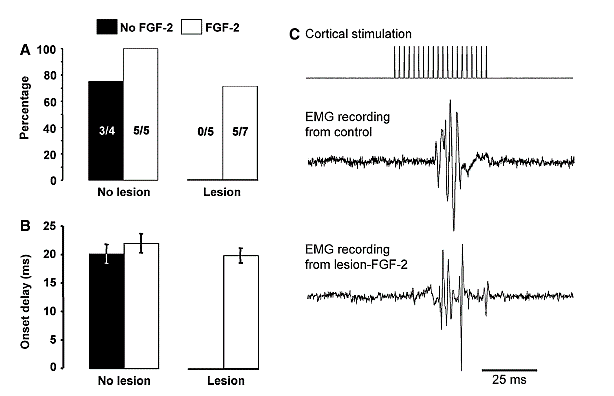
Several studies have shown that if ablation lesions are made from about P7- P12 there is spontaneous regeneration of the lost tissue, and this appears to result mostly from cells migrating to the lesion cavity from the subventricular zone [84]. Similar injuries from P1-5 or after P12 fail to do this. Other studies showed a similar effect of other midline ablation injuries such as the olfactory bulb or posterior cingulate cortex [85]. More lateral lesions do not show this spontaneous regeneration. Because FGF-2 stimulates neurogenesis in vitro it reasonable to examine the effects of subcutaneous injection of FGF-2 on neurogenesis in vivo. Monfils and her colleagues [15, 66] gave rats FGF-2 following neonatal motor cortex ablations and found that after injury at P10, but not at P3, there was regeneration of the lost tissue and the regenerated tissue was functional and showed normal connections to the spinal cord (Figure 5). Blockade of the regeneration by embryonic injections of Bromodeoxyuridine (BrdU) blocked the regeneration and functional recovery, just as it had in the spontaneous regeneration studies [15, 86].
Although there is a considerable literature on the effects of early-life nutrition on normal and abnormal behavioural development, relatively little is known about the role of early nutrition and recovery from perinatal brain injury. Most research on nutrients in development have focused on the effects of nutrient deficiencies especially related to protein-energy, iron, zinc, copper, and choline but Kaplan and her colleagues have emphasized the importance of using a combination of nutrients that would work synergistically to enhance metabolic activity, and ultimately brain functioning [87]. One promising product is EmpowerPlusTM. This product is a blend of 36 vitamins, minerals, and antioxidants and includes a proprietary blend of herbal supplements such as gingko bilboa and the amino acid precursors for neurotransmitters: choline, phenylalanine, glutamine, and methionine. Halliwell & Kolb fed this supplement mixed with regular rat chow to rat dams beginning at parturition and continuing until weaning, by which time the pups were also eating it [75]. Rats with P4 mPFC lesions showed marked improvement in both motor and cognitive functions and increased dendritic arbor in cortical pyramidal neurons. The mechanism of the dietary effect on neuronal structure may be epigenetic. For example, Dominguez-Salaz et al. studied gene methylation in the blood of infants conceived either in the Gambian dry or rainy season [88]. The maternal diets are dramatically different in the two seasons and so was the pattern of gene methylation.
More recently Shaw & Yager reported that feeding dams broccoli sprouts from E15 to P14, which is the best source of the powerful antioxidant and anti-inflammatory agent sulforaphane [52]. Sulforaphane acts to alter the pattern of gene expression in neurons and glia and is believed to influence normal brain development. Shaw & Yager used a model of placental insufficiency that leads to behavioral deficits and damage to the hippocampus and white matter. The offspring of the dams fed broccoli sprouts were protected from the behavioral and morphological abnormalities. This type of study is encouraging and should be expanded to other HI and perinatal ablation models.
Another diet-related treatment is the use of Docosahexaemoic acid (DHA), which is a dietary long-chain omega-3 polyunsaturated fatty acid with known neuroprotective properties. DHA modulates neuroinflammation, oxidative stress, and apoptosis and thus is a good candidate for treating HI. Several studies have now shown that DHA, and especially in combination with hypothermia, markedly improves functional outcome and reduces tissue injury after P7 HI [52].
Figure 5: EMG recordings from the wrist extensors following cortical stimulation in P10 motor cortex ablation rats that received FGF-2 injections. A) A summary of the number of rats in each group from which EMG activity could be measured in the wrist extensors. Lesion rats receiving no FGF-2 showed no EMG response whereas 5/7 lesion rats with FGF-2 did show normal EMG activity [66].
There are several proposed mechanisms for the treatment effects. In general, when there are beneficial treatment effects there are associate changes in neuronal morphology with increased dendritic length and/or spine density on cortical pyramidal neurons [34]. For example, these changes are seen in rats with other treatments after P4 mPFC ablation lesions including FGF-2, vitamin/mineral supplements, nicotine, or tactile stimulation [34]. Other studies have found spontaneous neurogenesis that regenerates the lost tissue or neurogenesis can be stimulated by subcutaneous injections of FGF-2 [14, 15, 66, 85]. There is also evidence that perinatal experiences such as stress or complex housing produce epigenetic modifications that correlate with functional outcomes so it is reasonable to predict that treatments for perinatal injuries will also induce epigenetic changes and this has been shown for pediatric closed head injuries in rats [89-91]. Given the evidence for inflammatory responses after HI injuries it seems likely that effective treatments could reduce postinjury inflammation although this has not been well studied to date.
Conclusions
The developing brain is responsive to a wide range of factors that modulate its development beginning with preconception experiences of the parents, gestational experiences, and postnatal experiences. There is a large literature showing how the developing brain and behavior can be influenced by such factors. The current standard of care for infants with perinatal brain injuries is hypothermia but the pre-clinical evidence suggests that much more could be done and some of the treatments, such as diet and extensive tactile stimulation both gestationally and postpartum, could be implemented clinically soon. Indeed, skin to skin contact with infants and caregivers (“kangaroo care”) is becoming more widely used with premature infants and could be used more widely by caregivers once the infants leave the hospital. The pre-clinical data show impressive effects on both behavior and brain. Other more invasive treatments, such as the pharmacotherapies, are more problematic but an understanding of the mechanisms underlying their effectiveness should be helpful.
It is known, for example, that tactile stimulation is effective and is correlated with increases spontaneous FGF-2 release in the skin. Thus, FGF-2 does not need to be given directly. Similarly, the dietary treatments are safe and easily implemented, examples being choline or broccoli sprouts (a source of sulforaphane). We have considered these factors as though they are independent singular events, but as we go through life experiences interact to alter both behaviour and brain, a process often referred to as metaplasticity. The field has only just begun to understand how different factors might interact with one another or how the effects of negative factors such as severe stress might be ameliorated by experiences such as tactile stimulation. Finally, the next step in translation to the clinic will be to demonstrate the efficacy of treatments in larger brain pre-clinical models of neonatal brain injury, including the use of kittens, fetal sheep, piglets, and nonhuman primates [70, 92-94].
Acknowledgements
The preparation of this manuscript was supported by a grant the Canadian Institutes for Advanced Research awarded to BK.
Conflicts of Interests
None.
Article Info
Article Type
Review ArticlePublication history
Received: Mon 16, Mar 2020Accepted: Tue 31, Mar 2020
Published: Tue 07, Apr 2020
Copyright
© 2023 Bryan Kolb. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2020.01.07
Author Info
Corresponding Author
Bryan KolbDepartment of Neuroscience, University of Lethbridge, Lethbridge, Alberta, Canada
Figures & Tables
Table 1: Factors positively affecting the synaptic organization and enhanced behavioural performance in otherwise normal animals.
|
Factor |
Example references |
|
[58] |
|
[35] |
|
[60] |
|
[40] |
|
[61] |
|
[62] |
|
[63] |
|
[64] |
|
[65] |
|
[66] |
|
[67] |
|
[68] |
Table 2: Factors improving behavior after surgical ablation or HI perinatal injury.
|
Factor |
Model |
Example References |
Outcome |
|
Behavioral therapies |
|||
|
SA |
[70] |
é vision |
|
SA |
[73] |
é vision |
|
HI |
[72] |
écognition, émotor |
|
SA |
[74] |
émotor |
|
SA |
[59] |
écognition, émotor |
|
HI |
[52] |
écognition, émotor |
|
SA |
[75] |
émotor |
|
HI |
[76] |
écognition, émotor |
|
Pharmacotherapies |
|||
|
SA |
[77] |
é vision |
|
SA |
[66] |
écognition, émotor |
|
|
SA |
[77] |
émotor |
|
HI |
[53] |
écognition, émotor |
|
HI |
[78] |
écognition, émotor |
|
SA |
[79] |
émotor |
|
HI |
[80] |
écognition, émotor |
|
HI |
[81] |
émotor |
|
SA |
[82] |
écognition |
|
HI |
[83] |
écognition |
écognition, émotor indicates improved performance on cognitive or motor tasks.
SA: surgical ablation; HI: hypoxic/ischemic. Treatments are given postinjury unless noted otherwise.
References
- Gale C, Statnikov Y, Jawad S, Uthaya SN, Modi N (2018) Neonatal brain injuries in England: population-based incidence derived from routinely recorded clinical data held in the National Neonatal Research Database. Arch Dis Child Fetal Neonatal Ed 103: F301-F306 [Crossref]
- Hagberg H, Edwards AD, Groenedndaal F (2016) Perinatal brain damage: The term infant. Neurobiol Dis 92: 102-112. [Crossref]
- Bale TL (2014) Lifetime stress experience: transgenerational epigenetics and germ cell programming. Dialogues Clin Neurosci 16: 297-305. [Crossref]
- Rodgers AB, Morgan CP, Leu NA, Bale TL (2015) Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A 112: 13699-13704. [Crossref]
- Song N, Liu J, An S, Nishino T, Hishikawa Y et al. (2011) Immunohistochemical analysis of histone H3 modifications in germ cells during mouse spermatogenesis. Acat Histochem Cytochem 44: 183-190. [Crossref]
- Mortera P, Herculano-Houzel S (2012) Age-related neuronal loss in the rat brain starts at the end of adolescence. Front Neuroanat 6: 45. [Crossref]
- Shankle WR, Landing BH, Rafii MS, Schiano A, Chen JM et al. (1998) Evidence for a postnatal doubling of neuron number in the developing human cerebral cortex between 15 months and 6 years. J Theor Biol 191: 115-140. [Crossref]
- Kolb B, Gibb R, Gorny G (2000) Cortical plasticity and the development of behavior after early frontal cortical injury. Dev Neuropsychol 18: 423-444. [Crossref]
- Bourgeois JP, Goldman-Rakic PS, Rakic P (1994) Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex 4: 78-96. [Crossref]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB et al. (2011) Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Nat Acad Sc U S A 108: 13281-13286. [Crossref]
- Blumberg MS, Freeman JH, Robinson SR (2010) A new frontier for developmental behavioral neuroscience. Oxford Handbook of Developmental Behavioral Neuroscience 1-6.
- Kolb B, Whishaw IQ (2015) Fundamentals of Human Neuropsychology 7th edition. New York: Worth.
- Lim DH, Mohajerani MH, LeDue J, Boyd J, Chen S et al. (2012) In vivo large-scale cortical mapping using channelrhodopsin-2 stimulation in transgenic mice reveals asymmetric and reciprocal relationships between cortical areas. Front Neural Circuits 6: 11. [Crossref]
- Kolb B, Gibb R, Gorny G, Whishaw IQ (1998) Possible regeneration of rat medial frontal cortex following neonatal frontal lesions Behav Brain Res 91: 127-141. [Crossref]
- Monfils MH, Driscoll I, Kamitakahara H, Wilson B, Flynn C et al. (2006) FGF-2 induced cell proliferation stimulates anatomical neurophysiological and functional recovery from neonatal motor cortex injury. Eur J Neurosci 24: 739-749. [Crossref]
- Kempermann G, Gage FH (1999) New nerve cells for the adult brain. Sci Am 28: 48-53. [Crossref]
- Greenough WT, Chang FF (1989) Plasticity of synapse structure and pattern in the cerebral cortex. Cerebral Cortex 7: 391-340.
- Finneman SJ, Nabulsi NB, Eid T, Detyniecki K, Lin SF et al. (2016) Imaging synaptic density in the living human brain. Science Transl Med 8: 348ra96. [Crossref]
- Wiesel TN, Hubel DH (1963) Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol 26: 1003-1017. [Crossref]
- Griffin F, Michell DE (1978) The rate of recovery of vision after early monocular deprivation in kittens. J Physiol 274: 511-537. [Crossref]
- Takesian AE, Hensch TK (2013) Balancing stability across brain development. Prog Brain Res 207: 3-34. [Crossref]
- Kolb B (2018) Brain plasticity in the adolescent brain. Manifestations and Mechanisms of Dynamic Brain Coordination over Development 25: 143-160.
- Salmason N, Jablonska B, Scafidi J, Vaccarino FM, Gallo V (2014) Neurobiology of premature brain injury. Nat Neurosc 17: 341-346. [Crossref]
- Rumajogee P, Bregman T, Miller SP, Yager JY, Fehlings MG (2016) Rodent hypoxia-ischemia models for cerebral palsy research: A systematic review. Front Neurol 7: 57. [Crossref]
- Fleiss B, Tann CJ, Degos V, Sigaut S, van Steenwinckel J et al. (2015) Inflammation-induced sensitization of the brain in term infants. Dev Med Child Neurol 57: 17-28. [Crossref]
- Khwaja O, Volpe JJ (2008) Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed 93: F153-F161. [Crossref]
- Mifsud G, Zammit C, Muscat R, Di Giovanni G, Valentino M (2014) Oligodendrocyte pathophysiology and treatment strategies in cerebral ischemia. CNS Neurosci Ther 20: 603-612. [Crossref]
- Vannucci RC, Vannucci SJ (2005) Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Dev Neurosci 27: 81-86. [Crossref]
- Eklind S, Mallard C, Leverin AL, Gilland E, Blomgrren K et al. (2001) Bacterial endotoxin sensitizes the immature brain to hypoxi-ischemic injury. Eur J Neurosci 13: 1101-1106. [Crossref]
- Kennard M (1938) Reorganization of motor function in the cerebral cortex of monkeys deprived of motor and premotor areas in infancy. J Neurophysiol 1: 477-496.
- Kennard M (1942) Cortical reorganization of motor function. Arch Neurol 48: 227-240.
- Passingham R, Perry H, Wilkinson F (1978) Failure to develop a precision grip in monkeys with unilateral neocortical lesions made in infancy. Brain Res 145: 410-414. [Crossref]
- Kolb B (1995) Brain plasticity and behavior. Hillsdale, NJ: Lawrence Erlbaum, 1995.
- Kolb B, Mychasiuk R, Muhammad A, Gibb R (2013) Brain plasticity in the developing brain. Prog Brain Res 207: 35-64. [Crossref]
- Williams P, Kolb B (2007) Stroke disrupts behavior and cortical motor map function. Soc Neurosc Abst 2007.
- Teuber HL (1975) Recovery of function after brain injury in man. In: Ciba Found Symp 1975: 159-190. [Crossref]
- Hebb DO (1947) The effects of early experience on problem solving at maturity. Am Psychol 2: 737-745.
- Morris RG (1949) Hebb DO: The Organization of Behavior, Wiley: New York; 1949. [Crossref]
- Stiles J (2012) The effects of injury to dynamic neural networks in the mature and developing brain. Dev Psychobiol 54: 343-349. [Crossref]
- Kolb B, Cioe J, Muirhead D (1998) Cerebral morphology and functional sparing after prenatal frontal cortex lesions in rats. Behav Brain Res 91: 143-155. [Crossref]
- Kolb B (1987) Recovery from early cortical damage in rats. I. Differential behavioral and anatomical effects of frontal lesions at different ages of neural maturation. Behav Brain Res 25: 205-220. [Crossref]
- Kolb B, Holmes C, Whishaw IQ (1987) Recovery from early cortical lesions in rats. III. Neonatal removal of posterior parietal cortex has greater behavioral and anatomical effects than similar removals in adulthood. Behav Brain Res 26: 119-137. [Crossref]
- Kolb B, Gibb R, van der Kooy D (1994) Neonatal frontal cortical lesions in rats alter cortical structure and connectivity. Brain Res 645: 85-97. [Crossref]
- Kolb B, Gibb R (1993) Possible anatomical basis of recovery of spatial learning after neonatal prefrontal lesions in rats. Behav Neurosc 107: 799-811. [Crossref]
- Kolb B, Whishaw IQ (1981) Neonatal frontal lesions in the rat: sparing of learned but not species-typical behavior in the presence of reduced brain weight and cortical thickness. J Comp Physiol Psychol 95: 863-879. [Crossref]
- Goldman PS, Galkin TW (1978) Prenatal removal of frontal association neocortex in the fetal rhesus monkey: anatomical and functional consequences. Brain Res 152: 451-485. [Crossref]
- Villablanca JR, Hovda DA, Jackson GF, Infante C (1993) Neurological and behavioral effects of a unilateral frontal cortical lesion in fetal kittens, II. Visual system tests and proposing a ‘critical period’ for lesion effects. Behav Brain Res 57: 72-92. [Crossref]
- Kolb B, Tomie JA (1988) Recovery from early cortical damage in rats. IV. Effects of hemidecortication at 1, 5, or 10 days of age. Behav Brain Res 28: 259-274. [Crossref]
- Kolb B, Gibb R, van der Kooy D (1992) Neonatal hemidecortication alters cortical and striatal structure and connectivity. J Comp Neurol 322: 311-324.
- Williams PT, Gharbawie OA, Kolb B, Kleim JA (2006) Experience-dependent amelioration of motor impairments in adulthood following neonatal medial frontal lesions in rats is accompanied by motor map expansion. Neurosc 141: 1315-1326. [Crossref]
- Hefter D, Marti HH, Gass P, Inta D (2018) Perinatal hypoxia and ischemia in animal models of schizophrenia. Front Psychiat 9: 106. [Crossref]
- Shaw OEF, Yager JY (2018) Preventing childhood and lifelong disability: maternal dietary supplementation for perinatal brain injury. Pharmacol Res 139: 228-242. [Crossref]
- Williams PTJA (2009) Neonatal stroke in rat impairs behaviour, anatomy, and neurophysiology in adulthood. Unpublished PhD thesis University of Lethbridge, AB, Canada.
- Williams PT, van Waes LT, Kolb B (2009) Reaching deficits are associated with abnormally large motor maps in adulthood following postnatal day 3 stroke in rats. Soc Neurosc Abst 738: 6.
- McEwen BS (2006) Protective and damaging effects of stress mediators: central role of the brain. Dialog Clin Neurosc 8: 367-381. [Crossref]
- McEwen BS, Morrison JH (2013) The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79: 16-29. [Crossref]
- Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE (2003) Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci U S A 100: 10523-10528. [Crossref]
- Sirevaag AM, Greenough WT (1988) A multivariate statistical summary of synaptic plasticity measures in rats exposed to complex, social, and individual environments. Brain Res 441: 386-392. [Crossref]
- Gibb R (2004) Perinatal experience and recovery from brain injury. Unpublished PhD Thesis University of Lethbridge, Lethbridge, AB, Canada.
- Richards S, Mychasiuk R, Kolb B, Gibb R (2012) Tactile stimulation during development alters behaviour and neuroanatomical organization of normal rats. Behav Brain Res 231: 86-91. [Crossref]
- Bell HC, Pellis SM, Kolb B (2010) Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortex. Behav Brain Res 207: 7-13. [Crossref]
- Greenough WT, Chang FF (1989) Plasticity of synapse structure and pattern in the cerebral cortex. Cerebral Cortex 7: 391-340.
- van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neurosc 2: 266-270. [Crossref]
- Muhammad A, Kolb B (2011) Prenatal tactile stimulation attenuates drug-induced behavioral sensitization, modifies behavior, and alters brain architecture. Brain Res 1400: 53-65. [Crossref]
- Robinson TE, Kolb B (2004) Structural plasticity associated with drugs of abuse. Neuropharmacol 47: 33-46. [Crossref]
- Monfils MH, Driscoll I, Vavrek R, Kolb B, Fouad K (2008) FGF-2 induced functional improvement from neonatal motor cortex injury via corticospinal projections. Exp Brain Res 185: 453-460. [Crossref]
- Meck WH, Williams CL (2003) Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosc Biobehav Rev 27: 385-399. [Crossref]
- Silasi G, Kolb B (2007) Chronic inhibition of cyclooxygenase-2 induces dendritic hypertrophy and limited functional improvement following motor cortex stroke. Neurosce 144: 1160-1168. [Crossref]
- Bland BH, Cooper RM (1969) Posterior neodecortication in the rat: age at operation and experience. J Comp Physiol Psych 69: 345-354. [Crossref]
- Cornwell P, Overman W (1981) Behavioral effects of early rearing conditions and neonatal lesions of the visual cortex in kittens. J Comp Physiol Psychol 95: 848-862. [Crossref]
- Rojas JJ, Deniz BF, Miguel PM, Diaz R, Hermel Edo et al. (2013) Effects of daily environmental enrichment on behavior and dendritic spine density in hippocampus following neonatal hypoxia-ischemia in the rat. Exp Neurol 241: 25-33. [Crossref]
- Schuch CP, Jeffers MS, Antonescu S, Nguemeni C, Gomez-Smith M et al. (2014) Enriched rehabilitation promotes motor recovery in rats exposed to neonatal hypoxia-ischemia. Behav Brain Res 304: 42-50. [Crossref]
- Comeau W, Gibb R, Hastings E, Cioe J, Kolb B (2008) Therapeutic effects of complex rearing or bFGF after perinatal frontal lesions. Dev Psychobiol 50: 134-146. [Crossref]
- Kolb B, Gibb R (2010) Tactile stimulation facilitates functional recovery and dendritic change after neonatal medial frontal or posterior parietal lesions in rats. Behav Brain Res 214: 115-120. [Crossref]
- Halliwell C, Kolb B (2003) Vitamin/mineral supplements enhance recovery from perinatal cortical lesions in rats. Soc Neurosc Abst 29: 459.11.
- Lin EP, Miles L, Hughes EA, McCann JC, Vorhees CV et al. (2014) A combination of mild hypothermia and sevoflurance affords long-term protection in a modified neonatal mouse model of cerebral hypoxia-ischemia. Anes Analges 119: 1158-1173. [Crossref]
- Comeau W, Hastings E, Kolb B (2007) Pre- and postnatal FGF-2 both facilitate recovery and alter cortical morphology following early medial prefrontal cortical injury. Behav Brain Res 180: 18-27. [Crossref]
- Gidyk DC, Williams P, Davidov DE, Kolb B (2009) Neonatal post-stroke nicotine treatment attenuates adulthood skilled reaching deficits and motor map dysfunction in rats. Soc Neurosc Abst 2009, 738.3.
- Kolb B, Gibb R, Pearce S, Tanguay R (2008) Prenatal exposure to prescription medications alters recovery following early brain injury in rats. Soc Neurosc Abstr 349.5.
- Berman DR, Liu Y, Barks J, Mozurkewich E (2010) Treatment of docosahexaenoic acid after hypoxia-ischemia improves forepaw placing in a rat model of perinatal hypoxia-ischemia. Am J Obstetrics Gynecol 203: 385.e1-e5. [Crossref]
- LaRosa DA, Ellery SJ, Snow RJ, Walker DW, Dickinson H (2016) Maternal creatine supplementation during pregnancy prevents acute and long-term deficits in skeletal muscle after birth asphyxia: a study of structure and function of hind limb muscle in the spiny mouse. Pediatr Res 80: 852-860. [Crossref]
- Kolb B, Halliwell C, Gibb R (2016) Nutritional and environmental influences on brain development: Critical periods of brain development, pathways, and mechanisms of effect. Nutrition and the developing brain London: CRC Press.
- Almli CR, Levy TJ, Han BH, Shah AR, Gidday JM et al. (2000) BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp Neurol 166: 99-114. [Crossref]
- Kolb B, Gibb R, Gorny G, Whishaw IQ (1998) Possible brain regrowth after cortical lesions in rats. Behav Brain Res 91: 127-141. [Crossref]
- Gonzalez CL, Gibb R, Kolb B (2002) Functional recovery and dendritic hypertrophy after posterior and complete cingulate lesions on postnatal day 10. Dev Psychobiol 40: 138-146. [Crossref]
- Kolb B, Pedersen B, Gibb R (2012) Embryonic pretreatment with bromodeoxyuridine blocks neurogenesis and functional recovery from perinatal frontal lesions in rats. Dev Neurosci 34: 228-239. [Crossref]
- Rucklidge JJ, Kaplan BJ (2013) Broad-spectrum micronutrient formulas for the treatment of psychiatric symptoms: a systematic review. Expert Rev Neurother 13: 49-73. [Crossref]
- Dominguez-Salas P, Moore SE, Baker MS, Bergen AW, Cox SE et al. (2014) Maternal nutrition at conception modulates DNA methylation of human metastable epialles. Nat Commun 5: 3746. [Crossref]
- Mychasiuk R, Ilnytskyy S, Kovalchuk O, Kolb B, Gibb R (2011). Intensity matters: brain, behaviour and the epigenome of prenatally stressed rats. Neurosc 180: 105-110. [Crossref]
- Mychasiuk R, Zahir S, Schmold N, Ilnytskyy S, Kovalchuk O et al. (2012) Parental enrichment and offspring development: modifications to brain, behavior and the epigenome. Behav Brain Res 228: 294-298. [Crossref]
- Mychasiuk R, Hehar H, Ma I, Kolb B, Esser MJ. (2015) The development of lasting impairments: a mild pediatric brain injury alters gene expression, dendritic morphology, and synaptic connectivity in the prefrontal cortex of rats. Neurosc 288: 145-155. [Crossref]
- Bennet L, Peebles DM, Edwards AD, Rios A, Hanson MA (1998) The cerebral hemodynamic response to asphyxia and hypoxia in the near-term fetal sheep as measured by near infrared spectroscopy. Pediatr Res 44: 951-957. [Crossref]
- Kyng KJ, Skajaa T, Kerrn-Jespersen S, Andreassen CS, Bennedsgaard K et al. (2015) A Piglet model of neonatal hypoxic-ischemic encephalopathy. J Vis Exp 99: e52454. [Crossref]
- Griffith JL, Shimony JS, Cousins SA, Rees SE, Mccurnin DC et al. (2012) MR imaging correlates of white-matter pathology in a preterm baboon model. Pediatr Res 71:185-191. [Crossref]
