A Novel Surgical Technique for Closure of Dehiscence Conjunctiva Due to Glaucoma Drainage Device
A B S T R A C T
Background: Step by step surgical technique for closure of dehisced conjunctiva in a patient with glaucoma drainage device (GDD).
Purpose: To describe a step by step new surgical technique using combined tutoplast, conjunctival autograft and amniotic membrane to close dehisced conjunctiva in a patient with GDD.
Case report: Patient with previous history of herpetic kerato uveitis and Baerveldt tube developed dehiscence of conjunctiva over the plate. He had previous surgical interventions on two occasions, which consisted of direct closure of the conjunctiva and conjunctival autograft, with no success. In order to close the persistent defect, he underwent additional surgery in the form of relieving conjunctival incisions, tutoplast, conjunctival autograft and amniotic membrane to ensure closure of defect and healing.
Conclusions: Tube/plate erosions are a very challenging problem to manage, especially in eyes that have undergone several surgical interventions. As demonstrated here, tutoplast, conjunctival autograft and amniotic membrane can be used in conjunction, in situations where conjunctival scarring otherwise preclude successful repair.
Keywords
Glaucoma drainage device, GDD, Baerveldt tube, conjunctival dehiscence, tutoplast, conjunctival autograft, amniotic membrane, tube extrusion, conjunctival erosion
Background
The increasing use of glaucoma drainage implants, as a surgical modality for the treatment of glaucoma is not without its limitations. The extrusion or ‘externernalization’ of such tubes or plates through an eroded conjunctiva characterises by far the most significant complication of such devices due to its high-risk factor for subsequent endopthalmitis [1]. Although causes for conjunctival erosion have been postulated including dehiscence of the suture, erosion of the scleral/graft patch or even mechanical factors exacerbated by micro-motion and ocular movements the complex nature of aetiology requires the operating surgeon to adapt their technique to ensure best outcomes [2]. To reduce the risk of tube exposure during glaucoma drainage device (GDD) the implanted tube is conventionally covered by a patch graft. However due to poor integration to host tissue and lack of cellular infiltration from the adjoining conjunctival stroma; there is a significant risk of advanced weakening and subsequent erosion. This may predispose the eye to sight threatening infection. The ideal patch graft should aim to offer the surgeon sufficient tensile strength, be appropriate for tectonic maintenance whilst simultaneously promoting cellular infiltration by the surrounding host conjunctival stroma [3]. This would reduce the gradual erosion of the allogeneic patch.
There however remains a significant paucity of literature with regards to the best method to resolve this challenging problem for glaucoma surgeons. Scarring at the implant site and poor conjunctival status due to chronic medication use, significantly reduces the success rate of direct closure often leading to repeated surgical attempts at repair. Incidence rates of tube exposure reported in literature vary between 2-3% for patients between the Ahmed, Baerveldt or Molteno implants [4]. Many different techniques have attempted to tackle this complication, varying from the use of various tissues for the graft: autologous donor sclera, pericardium, dura mater, amniotic membrane or attempting the modify GDD insertion technique and trying to create partial thickness scleral tunnel [2, 5, 6]. Other described techniques include double layer of amniotic membrane, forniceal conjunctival pedicle flap for repair of conjunctival deficient tube erosions and simply removing of the implant and placing a new drainage device. However, these are not without their limitations. We describe a technique successfully adopted by our institution when dealing with patients not responsive to conventional closure techniques. A combination of relieving conjunctival incisions, tutoplast, conjunctival autograft and amniotic membrane has been adopted with particularly successful outcomes.
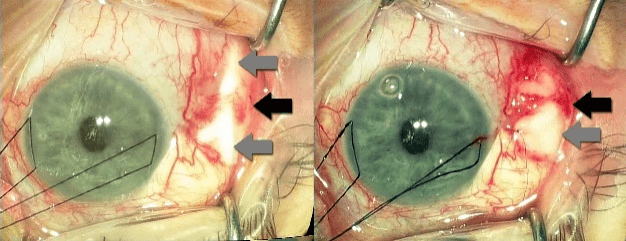
Figure 1: Left image: Grey arrows demonstrate conjunctival defects and the black arrow the bridging conjunctiva.
Right image: Arrow A shows the tutoplast graft tucked under the bridging conjunctiva (black arrow).
Case Example
A 72 years old male with history of herpetic kerato-uveitis, dry eyes, glaucoma for which he required Baerveldt tube surgery developed dehiscence of conjunctiva over the plate. Due to fragile nature of the tissue and lack of sufficient viable and mobile conjunctiva, the Baerveldt tube was placed in a position more anterior and temporal than described. Four weeks post-surgery he developed dehiscence of conjunctiva over the Baerveldt plate that did not heal with conventional closure, including direct closure of the conjunctiva and conjunctival autograft. A decision to implement our modified technique was made to subsequently treat the patient.
Figure 2: Left image: Arrow A: Area of conjunctival relieving incision.
Arrow B: Direct closure of the smaller conjunctival defect.
Right image: Arrow A: Site of conjunctival autograft harvest.
Arrow B: Direct closure of the smaller conjunctival defect. Arrow C: Closure of the larger conjunctival defect using conjunctival autograft.
Surgical Technique
An initial 7.0 silk corneal traction suture is placed in order to allow exposure of the surgical site (superior temporal region of the right eye of this case.). The areas of dehisced conjunctiva are identified and tutoplast patch graft is cut to size and carefully tucked under the bridging conjunctiva. The tutoplast is subsequently sutured to sclera with 10.0 nylon interrupted sutures. To enable direct closure of any smaller defects, conjunctival relieving incisions are made and 8.0 vicryl continuous sutures used for the closure of these defects. Conjunctival autograft is harvested from the inferior conjunctiva of the same eye and placed over the larger defect ensuring adequate coverage and sutured with 8.0 vicryl-interrupted sutures. A large piece of amniotic membrane is placed over the surgical area and sutured down to the conjunctiva with 8.0 vicryl. A large bandage contact lens is inserted at end of surgery. This technique prevented subsequent dehiscence over the surgical site.
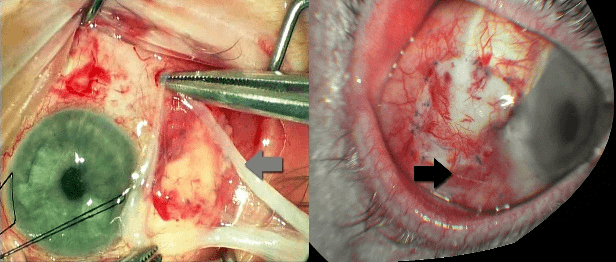
Figure 3: Left image: Amniotic membrane placed over the surgical area
Right image: One-week post surgery, black arrow shows bandage contact lens and successful healing of the conjunctiva.
Discussion
Tube erosion remains a surgical challenge for glaucoma surgeons. Risk factors include tube malposition, tissue turgor, mechanical rubbing of the eyelid margin against the tissue patch graft, excessive conjunctival tension over the tube, lack of a smooth tapered surface between the patch graft and the use of a high number of glaucoma medications before shunt implantation and inflammation [7]. Although complications can be anticipated, an optimum management technique has yet to be proven.
Although almost all glaucoma surgeons in the first instance attempt direct closure; in certain situations, conjunctival tissue cannot be advanced to cover the graft. Revision surgery commonly advocates covering the exposed area of tube with graft material and attempting to advance the conjunctiva. The uses of amniotic membrane, free conjunctival graft or buccal tissue have all been proposed as possible solutions. However, failure rates remain high leading to additional surgical intervention or in extreme cases, requiring explantation. However, there is no literature advocating the use of combination techniques to tackle this potentially sight threating complication. The Tutoplast is created by a scientific method involving virally inactiving, sterilising and preserving human tissue such that it can be used as an allograft [8]. It consists of low-profile collagen within a multi-directional matrix allowing for greater intraoperative handling. Its use has been described in literature for a vast array of ophthalmic surgery including corneal, oculoplastic and scleral buckle surgery [9, 10]. It provides essential support not simply tectonically but also as an epithelisation substrate.
Our modified technique describes a successful method that can be implemented even in the most complex of cases. Our case example illustrates its implementation in a patient with multifactorial risk factors. We have been able to demonstrate and apply a novel approach in our institution: tutoplast, conjunctival autograft and amniotic membrane, used in conjunction, in situations where conjunctival scarring otherwise precludes successful repair.
Article Info
Article Type
Case ReportPublication history
Received: Wed 20, Nov 2019Accepted: Thu 05, Dec 2019
Published: Fri 13, Dec 2019
Copyright
© 2023 Divya Mathews. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.AJSCR.2019.01.01
Author Info
Abdus Samad Ansari Divya Mathews
Corresponding Author
Divya MathewsStanley Eye Unit, Llanfair road, Abergele, United Kingdom
Figures & Tables
Right image: Arrow A shows the tutoplast graft tucked under the bridging conjunctiva (black arrow).

Arrow B: Direct closure of the smaller conjunctival defect.
Right image: Arrow A: Site of conjunctival autograft harvest.
Arrow B: Direct closure of the smaller conjunctival defect. Arrow C: Closure of the larger conjunctival defect using conjunctival autograft.
Right image: One-week post surgery, black arrow shows bandage contact lens and successful healing of the conjunctiva.
References
- Ranganath A, Hashim A (2011) Late-Onset Endophthalmitis Secondary to Exposed Glaucoma Tube Implant in a Rare Case of Paediatric Glaucoma. Case Rep Ophthalmol Med 2011: 183647. [Crossref]
- Oana S, Vila J (2012) Tube Exposure Repair. J Curr Glaucoma Pract 6: 139-142. [Crossref]
- Dubey S, Prasanth B, Acharya MC, Narula R (2013) Conjunctival erosion after glaucoma drainage device surgery: A feasible option. Indian J Ophthalmol 61: 355-357. [Crossref]
- Budenz DL, Barton K, Feuer WJ, Schiffman J, Costa VP et al. (2011) Treatment Outcomes in the Ahmed Baerveldt Comparison Study after One Year of Follow-up. Ophthalmology 118: 443-452. [Crossref]
- Lind JT, Shute TS, Sheybani A (2017) Patch graft materials for glaucoma tube implants. Curr Opin Ophthalmol 28: 194-198. [Crossref]
- Leong JK, McCluskey P, Lightman S, Towler HM (2006) Outcome of graft free Molteno tube insertion. Br J Ophthalmol 90: 501-505. [Crossref]
- Sarkisian SR Jr (2009) Tube shunt complications and their prevention. Curr Opin Ophthalmol 20: 126-130. [Crossref]
- Novitskaya ES, Clifford L, Vivian AJ (2013) Tutoplast pericardium patch graft for scleral thinning following strabismus surgery. Eye (Lond) 27: 682-683. [Crossref]
- Yoo C, Kang SY, Eom YS, Kim HM (2010) Temporary Repair of Corneal Perforation Using Tutoplast((R))-Processed Pericardium Graft. Ophthalmic Surg Lasers Imaging 2010: 1-3. [Crossref]
- Weissgold DJ, Millay RH, Bochow TA (2001) Rescue of exposed scleral buckles with cadaveric pericardial patch grafts. Ophthalmology 108: 753-758. [Crossref]
