Journals
A Noninvasive Method for Assessing Oocyte Competency
A B S T R A C T
There is an increasing demand to evaluate oocyte competency and viability for oocyte cryopreservation and use for in vitro fertilization. Oocytes suffer decreased survival of cryopreservation as compared to embryos, due to physical characteristics of the oocyte. The objective of this study is to determine if a specific gravity device (SGD) can estimate oocyte viability based on oocyte descent through the buoyancy system. All experiments were performed in a research laboratory with a randomized block design with repeated measures. Three hundred-seventy-six oocytes were collected from seven mice and randomly assigned to one of four treatments: exposure to 60°C for 30 min, acidified media for 1h, ethylene glycol-glycerol cryoprotectant for 1h, or standard culture to serve as control. To further analyze the relationship between oocyte descent time and viability, 98 additional oocytes were passed through SGD before and after treatment. Oocytes were stained with Coomassie Blue to determine membrane permeability and estimate viability based on treatment. Oocytes treated with 60°C heat, acidified media and ethylene glycol/ glycerol cryoprotectant solution demonstrated altered descent times from control and pre-treatment oocytes (P<0.05). Oocytes exposed to heat and cryoprotectants descended more rapidly through SGD than control and pre-treatment oocytes (P<0.05). Oocytes treated with acidified media descended more slowly through SGD (P<0.05). Permeation of stain into oocytes exposed to lethal treatments confirmed changes in membrane integrity post-treatment and further indicates SGD can detect such shifts. This suggests SGD can predict competency between live and dead oocytes. In conclusion, SGD can detect shifts in oocyte density due to altered membrane permeability, which can suggest information about oocyte competency. This information can help differentiate between high- and low-quality fresh oocytes to help select which oocytes to freeze and result in improved oocyte cryopreservation and fertilization.
Keywords
Oocyte competency, oocyte viability, oocyte buoyancy, noninvasive, specific gravity device
Introduction
Oocyte cryopreservation allows women to delay fertility for various medical or personal reasons. In animals, oocyte cryopreservation allows for female genetic storage. Additional advantages include cryobanking of oocytes for donation, preserving genetics of endangered species, and overcoming ethical issues and legal restrictions associated with embryo cryopreservation [1-8]. Despite the advantages associated with oocyte cryopreservation, maintaining oocyte competency throughout cryopreservation is challenging. Thus, improved methods to select for oocyte competency before freezing is warranted.
Oocyte quality can be indicated by cumulus cell association and adherence to the oocyte [9-12]. However, to improve fertilization, it is common practice to remove cumuli, either mechanically or enzymatically. Denuding the cumulus oophorous does not impact oocyte survival during cryopreservation [13]. Without intact cumulus cells, morphological analysis with light microscopy is the only current noninvasive method to predict the quality of an oocyte. High quality oocyte morphology has been indicated by a perfectly spherical shape, regular zona pellucida, and a translucent, homogeneously coloured cytoplasm without inclusions [14]. While oocyte morphology is helpful, it is often insufficient in determining quality as only 30-40% of cryopreserved, high quality bovine oocytes reach the blastocysts stage after fertilization [15]. These demands improvements in both freezing technologies and in preemptive selection techniques, which need to be simple and rapid to perform, inexpensive, reliable, and noninvasive.
The low success rate of cryopreserved oocytes is partially due to intracellular ice crystal formation lysing the cell and affecting its structural integrity [15, 16]. Oocyte cryopreservation can reduce oocyte viability by inducing premature extrusions of the cortical granules which harden the zona pellucida, induce volume changes which affect the structural integrity of the oolemma and dissemble the meiotic spindle, which can result in chromosomal abnormalities [17-20]. To prevent structural damages caused by intracellular ice formation, cryoprotectants (CP) are used to dehydrate the cell. Because the oocyte is one of the largest cells in the body and spherical, its low surface area to volume ratio and inherent concentration gradient of its spherical shape challenge the permeability of CP [1, 9].
The prolonged exposure to CP necessary to dehydrate the cell is often the cause of decreased oocyte viability, as CP can alter the intracellular pH, induce oxidative stress, cause chemical toxicity and osmotic shock [18, 20-22]. Oocytes are also more prone to chilling sensitivity due to their high cytoplasmic lipid content [18, 23]. These factors decrease oocyte viability after cryopreservation and cannot always be identified by morphological analysis. Therefore, new methods to detect oocyte viability before freezing are necessary for improved success of oocyte cryopreservation.
Previously, our lab created a method to detect embryo viability with a noninvasive embryo assessment technique (NEAT) using a specific gravity device (SGD) [24, 25]. Applying this technique to oocytes would allow further indication of oocyte viability, thus reducing time and energy spent freezing and thawing incompetent oocytes. The objective of the present study was to determine if the SGD can predict oocyte competency prior to cryopreservation. By studying oocyte buoyancy before and after exposure to lethal treatments, SGD efficacy in predicting oocyte viability was evaluated.
Materials and Methods
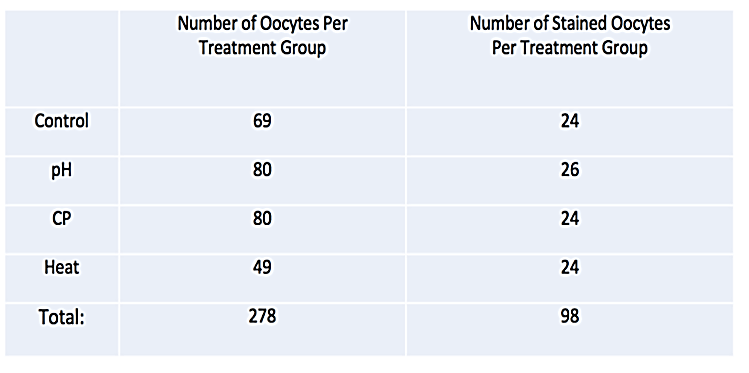
All experiments were approved by Texas Tech University Animal Care and Use Committee. Oocytes (n=278) were collected from twenty-two, 6-8-week-old mice, stimulated using standard protocol [25]. After micro-dissection from the oviduct and ovary, oocytes were randomly assigned to one of four treatment groups: Control, pH adjusted media, prolonged exposure to CP or treatment at 60°C (Table 1). All oocytes passed were assessed through SGD before treatment to establish initial descent time. After initial pass through SGD, the first group of oocytes were placed into standard culture conditions (36.9°C, 5.8% CO2, balance room air) to serve as control. Oocytes in other treatment groups were then either immersed in Modified Ham’s F-10 Media which has been adjusted to a pH of 6.0 from 7.4 with Hydrochloric Acid for 1 h, the F3 solution of the Global Blastocysts Fast Freeze kit for 1h or exposed to a temperature of 60C for a period of 30 min. Oocytes were then returned to normal culture conditions and passed back through SGD for final descent rate estimations. After all data were collected, comparisons were made between initial and post treatment descent times to establish if there were any changes in oocyte buoyancy over the course of treatment.
To further examine the relationship between oocyte viability and buoyancy, ninety-eight additional oocytes were passed through SGD. After initial descent times were measured, oocytes were randomly assigned to one of four treatment groups (Table 1). Treatments were performed as previously described and oocytes were dropped through SGD post-treatment. After final measurements through SGD, all oocytes were immersed in Coomassie blue post treatment for 1 minute and then flushed with PBS to determine oocyte membrane integrity and estimate viability. Comparisons were made based on the uptake of stain into oocyte and treatment group.
Statistical Analysis
All data were analysed using the Statistical Package for the Social Sciences (SPSS ver. 12; Chicago, IL). The basic analysis was a two-way analysis of variance of treatment by time using a P-value of 0.05 for significance. In case of significance by the original analysis, the differences within time or treatment were reanalysed with either Student’s t-test or one-way analysis of variance with Tukey’s means separation. A Chi Square analysis was used when appropriate.
Results
There was no significant difference between oocytes treated with F3 media, pH adjusted media and controls before treatment (Figure 1; P >0.05). Oocytes treated with acidified media had slower descent times than before treatment (Figure 2; P <0.001). When embryos were stained to verify effectiveness of kill treatment, 71% of control oocytes and 92% of acid treated oocytes appeared to have intact membranes, because the stain was not absorbed into the cell (Figure 3).
Figure 1: Mean oocyte descent time before treatment. Error bars represent standard error. Bars with different letters a–b represent differences between mean descent time (P <0.05).
Figure 2: Effect of pH Shift, Cryoprotectants and 60C Heat on descent time. Comparison of oocyte descent time between control, pre-treatment and post-treatment in oocytes treated with exposure to acidified media, F3 solution and 60C heat. Error bars represent standard error. Bars with different letters a-c represent differences in descent time (P> 0.05).
Conversely, oocytes treated with CP and heat had more rapid descent times through SGD than before treatment (Figure 2; P<0.001). Oocytes exposed to CP for 1h experienced drastic shifts in descent time; faster than both control and pre-treatment oocytes (Figure 2; P<0.001). 100% of desiccated oocytes with CP absorbed stain and were deemed to be non-viable. Before treatment, there was no significant difference between heat treated oocytes and control group (Figure 1; P>0.05). After heat treatment, oocytes displayed faster descent times than before treatment, but were not statistically significant from control oocyte descent times (Figure 2). Only 20.8% of heat treated oocytes appeared to maintain cell membrane integrity by not absorbing stain, suggesting the majority of oocytes were damaged in the treatment process (Figure 3).
Figure 3: Oocyte survival of treatment as determined by staining with Coomassie blue. Percent of oocytes in each treatment group (control, pH, CP and heat) which appeared to be live by not absorbing stain or those which appear to be dead because the cell did uptake stain.
Discussion
SGD was effective in detecting differences between pre-treatment and post-treatment descent times in each group. When oocytes were treated with pH adjusted culture media, oocytes demonstrated longer descent times than before treatment (Figure 2). While stain was not absorbed in the majority of oocytes cultured in acidic conditions, SGD demonstrated the ability to detect differences between viable and non-viable oocyte descent times. Denuded oocytes lack the ability to regulate cellular pH [26-28]. This can be contributed by lack of tight junctions between cells (as an oocyte is a single cell) and inactive regulatory mechanisms in oocytes [26, 29, 30, 31]. In oocytes, pH is known to affect cytoskeletal elements and mitochondrial localization, which are both correlated to developmental incompetence [32-34]. Decreased pH can denature proteins, which limits the effect of the membrane and the cell cannot regulate bidirectional leakage. For these reasons, it is likely oocytes exposed to acidified culture media lost the ability to regulate bidirectional leakage. Therefore, SGD can allow early estimation of oocyte viability because, water, being a smaller molecule than Coomassie Blue, can permeate and alter cellular density before the stain could permeate into the cell. This demonstrates the sensitivity of SGD to detect oocytes suffering from shifts in pH from those in healthy culture conditions based on oocyte density.
Oocytes treated with F3 solution for 1h had significantly faster descent times than fresh oocytes and controls (Figure 2; P<0.001). The cause for this is likely two-fold. First, the glycerol and ethylene glycol solution dehydrated the cell and reduced its radius, thus reducing resistance as the oocyte descended through the fluid filled chamber. Second, while water was removed from the cell, CP likely entered the cell, increasing cellular density. These phenomena can be supported by experiments performed by Paynter et al., in which shrink-swell experiments demonstrate oocytes initial non-spherical shrinkage after exposure to hypertonic glycerol CP solutions resulting in increased surface area [35]. Glycerol ethylene glycol have high molecular weights and can permeate into the membrane with long time exposure. This should contribute to the faster descent times observed in the CP treated oocytes [35, 36]. This suggests the SGD was effective in detecting the change in oocyte buoyancy after exposure to CP. Rapid descent times of CP treated oocytes through SGD likely represent decreased resistance and increased cellular density after CP exposure. Stain data supports this theory as 100% of CP treated oocytes absorbed Coomassie Blue. It is well documented CP alters both physical parameters of the oocyte (e.g. volume and surface area) and intracellular osmolality [37]. SGD was able to detect these changes by means of oocyte buoyancy and could differentiate between healthy and osmotically damaged oocytes.
With respect to heat treated oocytes, there was no significant difference between post-treatment and control descent times (Figure 2; P > 0.05). However, post-treatment descent times were more rapid than pre-treatment, indicating SGD can detect changes in buoyancy in oocytes damaged with high temperatures (Figure 2; P<0.05). While heat treated oocytes did not demonstrate as dramatic shifts in buoyancy as compared to other groups (mean descent time before treatment was 23.5s as compared to 16.82s after treatment), this observation resembles previous data in zygotes in which dramatic shifts after heat treatment were not observed until 24h [25].
This study suggest SGD is a non-invasive and quantitative measurement of oocyte competency, which can be used to supplement a morphological analysis to select only the highest quality oocytes to cryopreserve. Damaged cells undergo changes which are not always easily visually apparent. These osmotic changes can be detected by SGD and oocyte descent time can be used to select healthy oocytes. When selecting which oocytes are of highest quality, it appears that more average oocyte descent times within a maternal cohort should be considered competent over fast and slow counterparts (Figure 2). Measuring buoyancy of all oocytes in the cohort prior to freezing would allow specification of healthy oocytes.
As assisted reproductive technologies continue to grow, there will be an increased demand to freeze oocytes. Currently, all non-fertilized oocytes retrieved will be frozen whether or not they demonstrate high competency. Embryologists’ time, space in cryotanks and resources are spent freezing non-viable oocytes, which will never regain viability. If SGD can be used to select higher quality oocytes to freeze, thus reducing the number of non-viable oocytes preserved, time and resources can be conserved.
Conclusion
SGD detected differences in oocyte descent time before and after exposure to lethal treatments. This can be used as a further means to predict oocyte quality prior to cryopreservation. Descent times of control and pre-treatment oocytes appear to represent healthy oocytes and display more average buoyancy compared to post-treatment oocytes. This indicates oocytes outside at least one standard deviation from the mean are likely non-viable and further efforts should not be invested on them. Freezing fewer non-viable oocytes will save time and resources during freezing, thawing, fertilization and culture, and will increase fertilization rates of in vitro produced embryos.
Author Contributions
All four authors were significant contributors to the work and manuscript preparation on this project. SP developed the original concept and initial design of SGD. CW and LP refined the design prior to embryo testing. CW, MO, SP and LP designed experiment. CW performed testing with mouse embryos. While CW developed the original manuscript, all four were involved in editing for final content.
Acknowledgments
We greatly appreciate the support and feedback of Jessica Baggerman, Darvin Cuelllar, and Kelsea Brown.
Funding
The authors would like to thank the South Plains Foundation and the Laura W. Bush Institute for Women’s Health for funding of this project.
Conflicts of Interest
Cara Wessels has financial interest in Embryotics, LLC which has licensed this technology from Texas Tech University. All other authors have no conflict of interest.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 19, Jun 2020Accepted: Mon 29, Jun 2020
Published: Fri 03, Jul 2020
Copyright
© 2023 Cara Wessels Wells. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CROGR.2020.02.02
Author Info
Cara Wessels Wells L. Penrose M. Orth S. Prien
Corresponding Author
Cara Wessels WellsDepartment of Animal & Food Science, Texas Tech University, Lubbock, Texas, USA
Figures & Tables




References
- Kuwayama M (2007) Oocyte Cryopreservation. J Mammal Ova Res 24: 2-7. [Crossref]
- D Meirow (2000) Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol 169: 123-131. [Crossref]
- Elkin Lucena, Diana Patricia Bernal, Carolina Lucena, Alejandro Rojas, Abby Moran et al. (2006) Successful ongoing pregnancies after vitrification of oocytes. Fertil Steril 85: 108-111. [Crossref]
- K Y Cha, S Y Han, H M Chung, D H Choi, J M Lim et al. (2000) Pregnancies and deliveries after in vitro maturation culture followed by in vitro fertilization and embryo transfer without stimulation in women with polycystic ovary syndrome. Fertil Steril 73: 978-983. [Crossref]
- H M Chung, S W Hong, J M Lim, S H Lee, W T Cha (2000) In vitro blastocyst formation of human oocytes obtained from unstimulated and stimulated cycles after vitrification at various maturational stages. Fertil Steril 73: 545-551. [Crossref]
- James J Stachecki, Jacques Cohen (2004) An overview of oocyte cryopreservation. Reprod Biomed Online 9: 152-163.
- Masashige Kuwayama, Gábor Vajta, Osamu Kato, Stanley P Leibo (2005) Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online 11: 300-308. [Crossref]
- S J Paynter (2005) A rational approach to oocyte cryopreservation. Reprod Biomed Online 10: 578-586. [Crossref]
- G Coticchio, M A Bonu, A Borini, C Flamigni (2004) Oocyte Cryopreservation: A Biological Perspective. Eur J Obstet Gynecol Reprod Biol 155: S2-S7. [Crossref]
- G Ambrosini, A Andrisani, E Porcu, E Rebellato, A Revelli (2006) Oocyte Cryopreservation: State of Art. Reprod Toxicol 22: 250-262. [Crossref]
- A Cooper, S J Paynter, B J Fuller, R W Shaw (1998) Differential effects of cryopreservation on nuclear or cytoplasmic maturation in vitro in immature mouse oocytes from stimulated ovaries. Hum Reprod 13: 971-978. [Crossref]
- C J Ruppert-Lingham, S J Paynter, J Godfrey, B J Fuller, R W Shaw (2003) Developmental potential of murine germinal vesicle stage cumulus–oocyte complexes following exposure to dimethylsulphoxide or cryopreservation: loss of membrane integrity of cumulus cells after thawing. Hum Reprod 18: 392-398. [Crossref]
- R Fabbri, E Porcu, T Marsella, G Rocchetta, S Venturoli (2001) Human Oocyte Cryopreservation: new perspectives regarding oocyte survival. Hum Reprod 16: 411-416. [Crossref]
- Giovanni Coticchio, Elena Sereni, Lucia Serrao, Silvia Mazzone, Immacolata Iadarola et al. (2004) What Criteria for the Definition of Oocyte Quality? Ann N Y Acad Sci 1034: 132-144. [Crossref]
- I G F Goovaerts, J L M R Leroy, E P A Jorssen, P E J Bols (2010) Noninvasive bovine oocyte quality assessment: possibilities of a single oocyte culture. Theriogenology 74: 1509-1520. [Crossref]
- Shaw JM, Trouson A, Gardner D (1993) Handbook of in vitro Fertilization. Boca Raton, FL.CRC Press. [chapter 11]
- P Mazur, W F Rall, S P Leibo (1984) Kinetics of waterloss and likelihood of intracellular freezing in mouse ova: influence of the method of calculating the temperature dependence of water permeability. Cell Biophys 6: 197-213. [Crossref]
- A Arav (2014) Cryopreservation of oocytes and embryos. Theriogenology 81: 96-102. [Crossref]
- J Carroll, D G Whittingham, M J Wood, E Telfer, R G Gosden (1990) Extra-ovarian production of mature viable mouse oocytes from frozen primary follicles. J Reprod Fertil 90: 321-327. [Crossref]
- Andreas Mavrides, David Morroll (2002) Cryopreservation of bovine oocytes: is cryoloop vitrification the future to preserving the female gamete? Reprod Nutr Dev 42: 73-80. [Crossref]
- R Fabbri, E Porcu, T Marsella, M R Primavera, G Rocchetta et al. (2000) Technical Aspects of Oocyte Cryopreservation. Mol Cell Endocrinol 169: 39-42. [Crossref]
- M Damien, A A Luciano, J J Peluso (1990) Propanediol alters intracellular pH and developmental potential of mouse zygotes independently of volume change. Hum Reprod 5: 212-216. [Crossref]
- N A Ruffing, P L Steponkus, R E Pitt, J E Parks (1993) Osmometric behavior, hydraulic conductivity and incidence of intracellular ice formation in bovine oocytes at different developmental stages. Cryobiology 30: 562-580. [Crossref]
- Cara Wessels, Lindsay Penrose, Khaliq Ahmad, Samuel Prien (2017) Noninvasive embryo assessment technique based on buoyancy and its association with embryo survival after cryopreservation. Theriogenology 103: 169-172. [Crossref]
- S D Prien, C E Wessels, L L Penrose (2015) Preliminary Trials of a Specific Gravity Technique in the Determination of Early Embryo Growth Potential. Hum Reprod 30: 2076-2083. [Crossref]
- Jason E Swain (2010) Optimizing the Culture Environment in the IVF Laboratory: Impact of PH and Buffer Capacity on Gamete and Embryo Quality. Reprod BioMed Online 21: 6-16. [Crossref]
- Seref Erdogan, Greg FitzHarris, Alina P Tartia, Jay M Baltz (2005) Mechanisms regulating intracellular pH are activated during growth of the mouse oocyte coincident with acquisition of meiotic competence. Dev Biol 286: 352-360. [Crossref]
- Karen P Phillips, Mary Ann F Petrunewich, Jennifer L Collins, Jay M Baltz (2002) The intracellular pH-regulatory HCO3/Cl exchanger in the mouse oocyte is inactivated during first meiotic metaphase and reactivated after egg activation via the MAP kinase pathway. Mol Biol Cell 13: 3800-3810. [Crossref]
- C A Gibb, P Poronnik, M L Day, D I Cook (1997) Control of Cytosolic pH in Two-Cell Mouse Embryos: Roles of H(+)-lactate Cotransport and Na+/H+ Exchange. Am J Physiol 273: C404-C419. [Crossref]
- François Hérubel, Said El Mouatassim, Pierre Guérin, René Frydman, Yves Ménézo (2002) Genetic expression of monocarboxylate transporters during human and murine oocyte maturation and early embryonic development. Zygote 10: 175-181. [Crossref]
- L J Edwards, D A Williams, D K Gardner (1998) Intracellular pH of the mouse preimplantation embryo: amino acids act as buffers of intracellular pH. Hum Reprod 13: 3441-3448. [Crossref]
- J M Squirrell, M Lane, B D Bavister (2001) Altering intracellular pH disrupts development and cellular organization in preimplantation hamster embryos. Biol Reprod 64: 1845-1854. [Crossref]
- B D Bavister, J M Squirrell (2000) Mitochondrial distribution and function in oocytes and early embryos. Hum Reprod 15: 189-198. [Crossref]
- Seiichiro Nagai, Tadashi Mabuchi, Shuji Hirata, Tomoko Shoda, Tsuyoshi Kasai et al. (2006) Correlation of abnormal mitochondrial distribution in mouse oocytes with reduced developmental competence. Tohoku J Exp Med 210: 137-144. [Crossref]
- S J Paynter, J J McGrath, B J Fuller, R W Shaw (1999) A Method for Differentiating Nonunique Estimates of Membrane Transport Properties: Mature Mouse Oocytes Exposed to Glycerol. Cryobiology 39: 205-214. [Crossref]
- Claudia Yamada, Heloísa Vasconcellos Amaral Caetano, Renata Simões, Alessandra Corallo Nicacio, Weber Beringui Feitosa et al. (2007) Immature Bovine Oocyte Cryopreservation: Comparison of Different Associations with Ethylene Glycol, Glycerol and Dimethylsulfoxide. Anim Reprod Sci 99: 384-388. [Crossref]
- Jun Liu, Steve Mullen, Qinggang Meng, John Critser, Andras Dinnyes (2009) Determination of oocyte membrane permeability coefficients and their application to cryopreservation in a rabbit model. Cryobiology 59: 127-134. [Crossref]
