A Case of Diffuse Multicentric Metachronous Astrocytoma of Temporoinsular and Intramedullary Location
A B S T R A C T
Multiple gliomas are rare glial tumors with a histology that is typically consistent with high-grade gliomas. A distinction is made between multifocal and multicentric gliomas according to criteria of anatomical continuity, as well as between synchronous and metachronous gliomas according to chronological time of onset. We present the case of a professional saxophonist with a left temporoinsular lesion who underwent awake craniotomy with monitoring of verbal and musical ability as well as primary sensory and motor cortices. Histopathological study revealed an isocitrate dehydrogenase 1 (IDH)-mutant diffuse astrocytoma. After 4 years of complete oncological remission, the patient developed impaired proprioception in all four extremities. An intramedullary lesion was detected at the level of C4 consistent with an IDH wild-type diffuse astrocytoma. We highlight the singularity of this case as it involved two low-grade glial lesions, separated in time (metachronous) and location (multicentric), as well as genetic differences between both lesions (IDH mutant and wild type).
Keywords
Diffuse astrocytoma, multicentric glioma, metachronous glioma, intramedullary astrocytoma
Introduction
Multiple gliomas are a relatively rare heterogeneous entity with histology consistent with high-grade gliomas in most cases. Among these, we can differentiate between multifocal glioma and multicentric glioma. In 1963, Batzdorf and Malamud proposed the criteria for making this distinction. In addition, according to the time of presentation, multiple gliomas can be defined as synchronous when they are simultaneously present on the first radiological examination and metachronous when months or years elapse between the diagnosis of both lesions. We present the case of a patient treated for a diffuse left temporoinsular astrocytoma with oncological criteria of complete remission who, three years later, underwent surgery for a low-grade cervical intramedullary astrocytoma.
Case Report
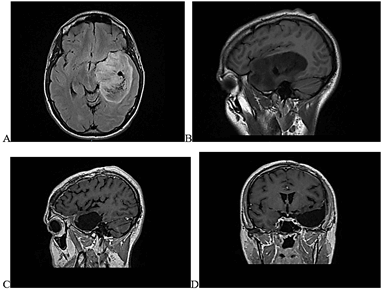
A 27-year-old male saxophonist with no significant medical history presented with absence seizures lasting seconds. He was examined in the Neurology Department and referred to Neurosurgery after the detection of a left temporoinsular space-occupying lesion. Examination showed no focal neurological deficits. Macroscopically complete resection was achieved through awake craniotomy with monitoring of verbal and musical abilities (the patient played the saxophone in the operating room during the mapping and resection phase) as well as motor and sensory cortices. Figure 1 shows pre and postsurgical MRI of temporoinsular lesion. Pathological study identified a diffuse astrocytoma (World Health Organization [WHO] grade II) with an isocitrate dehydrogenase 1 (IDH1) R132H mutation, MGMT methylated and ATRX loss. Immediate postoperative magnetic resonance imaging (MRI) revealed apparently complete resection. However, follow-up MRI one month after surgery showed possible residual tumor of the insular and external capsule region, shown to be neoplastic on methionine positron emission tomography. Adjuvant treatment with fractionated stereotactic radiotherapy and sequential PCV chemotherapy was therefore given. Subsequent follow-up MRIs performed every 6 months showed the remaining millimetric tumor remnant to be stable according to RANO criteria and response was considered to be oncologically complete.
Figure 1: Pre- and post-surgical MRIs showing apparently complete resection of temporoinsular lesion. A) Presurgical axial FLAIR images B) Presurgical sagittal T1-weigthed images. C) Postsurgical sagittal T1-weighted postcontrast images. D) Postsurgical coronal T1-weighted postcontrast images.
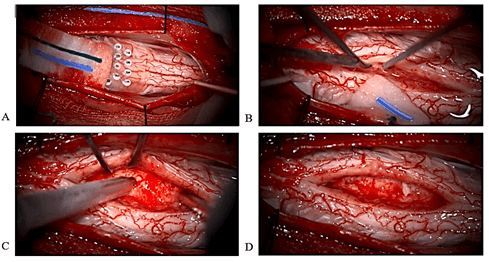
Figure 2: Operative images of intramedullary cervical lesion. A) Intraoperative dorsal column monitoring is used for finding location of midline myelotomy. B) Interface between the tumor and the cord is searched to determine the feasibility of resection. C) Ultrasonic aspirator gives soft tumor decompression. D) Apparently gross total resection is reached.
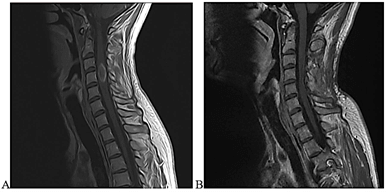
Four years after the initial surgery, the patient was seen due to the onset of numbness in the fingers and toes, with impaired proprioceptive sensitivity (unable to play the saxophone or tie his shoes) with neurological examination revealing hyperreflexia in all four extremities. Cervical and thoracic MRI showed a ring-enhancing, intramedullary, space-occupying lesion at C4. The patient was admitted and underwent laminoplasty and resection of the lesion. Intraoperative images are shown in (Figure 2). Complete resection was confirmed on post-surgical control MRI. Figure 3 shows presurgical and post-surgical MRI images of cervical lesion. The patient developed slight left hemiparesis (4/5) from which he recovered in the postoperative period. After several months, he continued to experience a slight alteration in proprioceptive sensitivity in all four extremities, which did not prevent him from carrying out his activities as a musician. Pathological analysis revealed an IDH wild-type diffuse astrocytoma (WHO grade II), preserved ATRX, MGMT methylated and BRAF negative. Cervical MRI 6 months after surgery confirmed complete tumor resection.
Figure 3: Pre- and post-surgical images of cervical lesion. A) Presurgical sagittal T1-weighted postcontrast images. B) Postsurgical sagittal T1-weighted postcontrast images show complete resection 6 months after surgery.
Discussion
Diffuse astrocytoma, classified as grade II according to the WHO classification of tumors of the central nervous system, is characterized by diffuse infiltration of the brain parenchyma, causing swelling and distortion (but not destruction) of the anatomical structures affected [1]. They represent 11-15% of all astrocytic brain tumors and usually affect adults, with a mean age of 36 years [2]. In adulthood, a disease-free rate of 34% is achieved at 10 years [3]. The standard of care continues to be maximal possible resection without causing neurological deficits [4]. Notable among the factors of poor prognosis is multicentricity, although most multifocal and multicentric gliomas are associated with more aggressive histologies such as glioblastomas (WHO grade IV) and/or anaplastic astrocytomas (WHO grade III) [5].
The difference between multifocal and multicentric glioma was defined by Batzdorf and Malamud: Multifocal glioma is a multiple glioma resulting from the spread or growth of the tumor along a known anatomical pathway [6]. These include white matter tracts or commissures (corpus callosum, fornix, internal capsule or massa intermedia), cerebrospinal fluid (CSF) channels or satellite or local metastases. In contrast, multicentric gliomas are multiple gliomas the components of which appear in different lobes or hemispheres with no known connecting anatomical pathway. These also include metachronous gliomas, which occur at different time points during the course of treatment [7]. Literature on multiple low-grade gliomas is very limited [8, 9]. The case we describe is even more atypical given the wide separation between the lesions - supratentorial intracranial and intramedullary. One of the largest series in the literature describes 25 cases of multicentric gliomas, in which the most frequent histology was glioblastoma multiforme (GBM, 48%), followed by anaplastic astrocytoma (20%), and simultaneous GBM and anaplastic astrocytoma (20%) [10]. In this series, only one case of diffuse glioma coexisting with GBM was found. No multicentric glioma with a medullary component is described. Only 4 of the 25 cases were metachronous, with a time interval of 2, 3 and 9 months and 6 years between both lesions, respectively. In our case, 4 years elapsed between the two lesions.
In the article by Inoue et al., which reports a case of supratentorial and infratentorial multicentric gliomas, the authors state that after reviewing the literature they found only 11 cases of these multicentric gliomas, and each presented a different histology [11-19]. In our case, although the histological grade was similar, the genetic profile was different. The temporoinsular astrocytoma was consistent with an IDH-mutant (R132H) grade II diffuse astrocytoma with loss of ATRX, p53 of 80% and Ki67 of 25% on immunohistochemistry. The intramedullary astrocytoma was classified as IDH wild type (negative molecular biology for IDH1 and IDH2 mutations), preserved ATRX, Ki67 of 1%, negative BRAF and weak p53 on immunohistochemistry. Methylation was present in both samples and telomerase reverse transcriptase immunoexpression analysis was negative. Diffuse astrocytomas in adulthood without IDH mutations, regardless of their histological grade, tend to be more aggressive [20].
The pathogenesis of multicentric tumors is yet to be elucidated. It is attributed to the ability of tumor cells to invade and migrate over long distances [5]. Willis et al. suggest that multicentric lesions arise as a result of a two-step process [21]. First, initiation occurs, a process by which a large area of the parenchymal brain or the entire brain undergoes a neoplastic transformation, making it more susceptible to uncontrolled neoplastic growth. Second, promotion takes place, by which neoplastic proliferation begins in different areas of the central nervous system after receiving different types of stimuli: biochemical, hormonal, mechanical or viral. Several cases of glial cell tumor metastasis have also been described in the literature, specifically, forms of CSF metastasis in patients with GBM. In autopsy studies, CSF dissemination of GBM is found in 15-25% of supratentorial sites and up to 60% of infratentorial sites [22]. Metastases are most commonly found in the leptomeningeal space and have a predilection for the upper lumbar or lumbosacral area [23]. In our case, the lesion was entirely intramedullary in nature, and the study of the rest of the neuronal axis found no other lesions, making the possibility of dissemination through the CSF very unlikely.
Conclusion
The singularity of the case presented lies in the appearance of two tumor lesions separated in time (metachronous) and spatial location (multicentric). Similarly, although multicentricity is most frequently associated with high-grade astrocytomas, our case had a low histological grade. In addition, there were differences in the genetics of both lesions: the intracerebral tumor was IDH mutant, and the intramedullary tumor was IDH wild type. The low prevalence of cases of this type makes it unfeasible from a cost-benefit perspective to routinely perform neural axis screening that could detect possible multicentricity before symptoms occur. However, we consider that careful clinical follow-up is essential. The patient being followed should be examined and queried about other possible clinical signs or symptoms that could lead to the appearance of this phenomenon.
Author Contributions
Not applicable.
Consent
Consent to participate was not required, as it is a retrospective case report.
Funding
FIMABIS (Andalusian Public Foundation for Biomedicine and Health research of Málaga) financed the translation of the article from Spanish to English.
Conflicts of Interest
None.
Availability of Data and Materials
Not applicable.
Code Availability
Not applicable.
Ethical Approval
Not applicable.
Article Info
Article Type
Case ReportPublication history
Received: Thu 28, Jan 2021Accepted: Mon 23, Aug 2021
Published: Mon 30, Aug 2021
Copyright
© 2023 Jorge Linares Torres. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSCR.2021.01.02
Author Info
Jorge Linares Torres Guillermo Ibanez Botella Antonio Selfa Rodriguez Laura Cerro Larrazabal Julia Casado Ruiz Miguel Angel Arraez Sanchez
Corresponding Author
Jorge Linares TorresNeurosurgery Department, Regional University Hospital of Malaga, University of Malaga, Malaga, Spain
Figures & Tables
References
1. Louis DN, Perry A,
Reifenberger G, von Deimling A, Figarella Branger D et al. (2016) The 2016
World Health Organization Classification of Tumors of the Central Nervous
System: a summary. Acta Neuropathol 131: 803-820. [Crossref]
2. Ostrom QT, de Blank
PM, Kruchko C, Petersen CM, Liao P et al. (2015) Alex’s Lemonade Stand
Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors
Diagnosed in the United States in 2007-2011. Neuro Oncol 16: x1- x36. [Crossref]
3. Nitta M, Muragaki
Y, Maruyama T, Iseki H, Ikuta S et al. (2013) Updated therapeutic strategy for
adult low-grade glioma stratified by resection and tumor subtype. Neurol Med
Chir (Tokyo) 53: 447-454. [Crossref]
4. Youland RS, Brown
PD, Giannini C, Parney IF, Uhm JH et al. (2013) Adult low-grade glioma: 19-year
experience at a single institution. Am J Clin Oncol 36: 612-619. [Crossref]
5. Patil CG, Eboli P,
Hu J (2012) Management of multifocal and multicentric gliomas. Neurosurg
Clin N Am 23: 343-350. [Crossref]
6. BATZDORF U, MALAMUD
N (1963) THE PROBLEM OF MULTICENTRIC GLIOMAS. J Neurosurg 20: 122-136. [Crossref]
7. Russell DS,
Rubinstein LJ (1977) Pathology of tumors of the nervous system, 4th ed. Williams
and Wilkins, Baltimore 240-242.
8. Sridharan V,
Urbanski LM, Bi WL, Thistle K, Miller MB et al. (2015) Multicentric Low-Grade
Gliomas. World Neurosurg 84: 1045-1050. [Crossref]
9. Terakawa Y,
Yordanova YN, Tate MC, Duffau H (2013) Surgical management of multicentric diffuse
low-grade gliomas: functional and oncological outcomes: clinical article. J
Neurosurg 118: 1169-1175. [Crossref]
10. Salvati M, Caroli E, Orlando ER, Frati A, Artizzu S et al. (2003) Multicentric
glioma: our experience in 25 patients and critical review of the literature. Neurosurg
Rev 26: 275-279. [Crossref]
11. Inoue A, Ohnishi T,
Kohno S, Mizuno Y, Kitazawa R et al. (2016) A case of multicentric gliomas in
both supra- and infratentorial regions with different histology: a case report.
World J Surg Oncol 14: 152. [Crossref]
12. Bradley WL (1880)
Case of gliosarcomatose tumours of the cerebrum and cerebellum. Pro Conn Med
Soc 2: 39-41.
13. Nakase H, Hisanaga
M, Iwanaga H (1987) An autopsy case of multicentric glioma of multiple
histopathology. No Shinkei Geka 15: 1073-1077. [Crossref]
14. Kotwica Z, Papierz
W (1992) Cerebral and cerebellar glial tumors in the same individual. Neurosurgery
30: 439-441. [Crossref]
15. Pell MF, Revesz T,
Thomas DG (1991) Multicentric malignant glioma. Br J Neurosurg 5:
631-634. [Crossref]
16. Mishra HB, Haran
RP, Singh JP, Joseph T (1990) Multicentric gliomas: two case reports and a
review of the literature. Br J Neurosurg 4: 535-539. [Crossref]
17. Misra BK, Steers
AJ, Miller JD, Gordon A (1988) Multicentric glioma presenting with hemorrhage. Surg
Neurol 29: 73-76. [Crossref]
18. SOLITARE GB (1962)
Cerebellar and cerebral gliomas occurring in the same individual. J
Neurosurg 19: 1079-1084. [Crossref]
19. Solomon A, Perret
GE, McCormick WF (1969) Multicentric gliomas of the cerebral and cerebellar
hemispheres: Case report. J Neurosurg 31: 87-93. [Crossref]
20. Gorovets D, Kannan
K, Shen R, Kastenhuber ER, Islamdoust N et al. (2012) IDH mutation and
neuroglial developmental features define clinically distinct subclasses of
lower grade diffuse astrocytic glioma. Clin Cancer Res 18: 2490-2501. [Crossref]
21. Willis RA (1960)
Pathology of Tumors, 4th ed. Butterworth London 811.
22. Vertosick Jr FT, Selker RG (1990) Brain stem and spinal metastases of supratentorial glioblastoma multiforme: a clinical series. Neurosurgery 27: 516-521. [Crossref]
23. Hamilton MG, Tranmer BI, Hagen NA (1993) Supratentorial glioblastoma with spinal cord intramedullary metastasis. Can J Neurol Sci 20: 65-68. [Crossref]
