1-(4-Methoxyphenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one: A Diphenyl Chalcone Derivative with Potent Antitumor Activity Via Up-Regulation of p21 Gene
A B S T R A C T
Background and Aim: Cancer is a disease of complex aetiology and is characterised by uncontrolled growth of abnormal cells. It is a major worldwide health problem. Many natural and synthetic chalcone or their derivatives showed anticancer activities. The aim of the present study is to evaluate the anticancer activity of novel chalcone derivatives and also to establish possible mechanism of action.
Materials and Methods: A series of chalcones 3-(3-phenoxyphenyl)-1-phenylprop-2-en-1-one (2a); 1-(4-chlorophenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one (2b); 1-(4-fluorophenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one (2c); 1-(4-Nitro-phenyl)-3-(3-phenoxy-phenyl)prop-2-en-1-one (2d); 1-(4-methoxyphenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one(2e) were evaluated for the cytotoxic activity both in vitro and in vivo. The in vivo antitumor activity of these compounds was estimated on Daltons Ascites Lymphoma induced solid tumor model. The effect of promising compound was further analysed by flow cytometer and RT- PCR analysis.
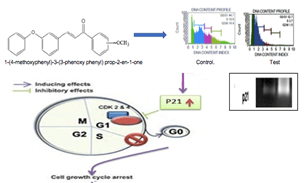
Results and Conclusion: 1-(4-methoxyphenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one and 1-(4- chlorophenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one was showed in vitro cytotoxic activity, DNA damage and antiproliferative activity. DLA induced solid tumor model suggested that 1-(4-methoxyphenyl)-3-(3- phenoxy phenyl) prop-2-en-1-one significantly reduced the tumor volume, increase the percentage tumor inhibition and reverse the haematological parameters. Flow cytometry analysis concluded that the compound induces cell cycle arrest at G0/G1 phase due to the over expression of p21. 1-(4-methoxyphenyl)-3-(3- phenoxy phenyl) prop-2-en-1-one may be a potential agent for cancer treatment.
Keywords
Dalton’s lymphoma ascites, Diphenyl chalcones, p21 gene expression, 1-(4-methoxyphenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one, DNA content analysis
Graphical Abstract
Introduction
Cancer has become one of the most lethal and detrimental diseases around the globe. It is a set of diseases epitomized by unrestrained growth and expansion of abnormal cells. Universally, there are 10 million new cases of cancer per annum [1]. According to IARC (International Agency for Research on Cancer) in 2018, the estimates of cancer affliction worldwide were 15.1 million new cases and 9.6 million cancer deaths [2]. Cancer has exhibited an unprecedented growth in the past few decades, and it was noted that around 70% of cancer deaths occur in low and middle-income countries and by 2030 about 11.5 million deaths is estimated [3]. In India, the highest fraction of cancer was stomach, breast, lung, oral cavity, lip etc. The cancer prevalence rate in India amplified by 28·2% and it was utmost in Kerala and Mizoram, followed by Haryana, Delhi, Karnataka, Goa, Himachal Pradesh, Uttarakhand, and Assam. Age-categorized incidence rates were topmost in the northeast states of Mizoram, Meghalaya, Arunachal Pradesh, and Assam [4]. Enormous resources are being invested in treatment, prevention and diagnosis of cancer. Synthetic organic chemistry has always played a crucial role in anticancer drug development. Development of drugs like 6-mercaptopurine, cytosine arabinoside, 5-fluorouracil, methotrexate, nitrogen mustards and cyclophosphamide were enabled due to the knowledge of DNA metabolism [5].
Even though notable progress has been made by the medical science, the accessibility of safe and specific anticancer drugs has remained a major challenge in clinical practice. The development of tumor resistance against available treatment modes like radiotherapy, chemotherapy together with patient noncompliance raises the demands for the introduction of newer drugs in cancer management [6]. Considering these points, a number of natural and synthetic molecules/compounds has been studied for cancer drug discovery and some of them were found promising. Chalcones possesses multiple biological and medicinal properties. Chalcones constitute one of the significant classes of anticancer agents that have shown ensuring therapeutic efficacy in the management of human cancers [7]. They are considered as the precursors of flavonoids and iso flavonoids and are profusely available in edible plants [8]. They show a wide variety of activities, including anticancer, anti-inflammatory and anti-parasitic activities. A number of reformations on chalcone chromophore have been reported including hydroxyl, methoxy, and amino groups as substituents with promising anticancer activity [9].
Clinical studies had indicated the remarkable bioavailability and maximum tolerance in the human body. Therefore, researchers focussing on synthesis of different chalcone analogues for the development of novel and more potent drugs for the treatment of numerous lethal diseases such as cancer, diabetes, HIV, TB, malaria, etc [7]. The ease of preparation, the potential of oral administration, and safety also support the feasibility of chalcone-based compounds as therapeutic agents [10]. Chalcones are the promising agents for the treatment of cancer because of their ability to block the NF-κB activation, induce apoptosis, and to inhibit proliferation, invasion, metastasis and angiogenesis. So, these natural and synthetic chalcones may serve as lead compound for cancer drug development [11]. In the present study, a series of novel chalcones were evaluated for its cytotoxicity both in vivo and in vitro, and also to establish the possible mechanism of action using analysis of DNA content, cell cycle analysis and gene expression studies.
Materials and Methods
I Chemicals and Reagents
Dulbecco's Modified Eagle’s Medium (DMEM), DMSO and Tris- EDTA was purchased from Sigma-Aldrich, USA; foetal bovine serum (FBS) and TRIzol® reagent (15596018) was obtained from Invitrogen Bio Services India Pvt. Ltd., Bangalore, India. Tissue culture flasks and other accessories were attained from Tarson's products Pvt. Ltd. Bangalore, India. Verso One step RT-PCR kit was acquired from Thermo-scientific, USA, while Brine shrimp eggs was obtained from Cochin Marine and Fisheries Research Institute Cochin, India.
II Cell Lines and Maintenance
HeLa (Human cervical carcinoma, tumorigenic and invasive), HaCat (Human Keratinocyte Immortalised) was initially purchased from NCCS, Pune. The cell was cultured in 25 cm2 tissue culture flask with DMEM supplemented with 10% FBS, L-glutamine, sodium bicarbonate and antibiotic solution containing: Penicillin (100 U/mL), Streptomycin (100 µg/mL), and Amphotericin B (2.5 µg/mL). Cultured cells were kept at 37ºC in a humidified 5% CO2 incubator (NBS Eppendorf, Germany). Daltons Lymphoma Ascites (DLA) cell line was obtained through the courtesy of Amala Cancer Research Center, Trissur, Kerala.
III Test Material
A series of chalcones 3-(3-phenoxyphenyl)-1-phenylprop-2-en-1-one (2a); 1-(4-chlorophenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one (2b); 1-(4-fluorophenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one (2c); 1-(4-Nitro-phenyl)-3-(3-phenoxy-phenyl)prop-2-en-1-one (2d); 1-(4-methoxyphenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one(2e) were evaluated for the cytotoxic activity both in vitro and in vivo [12].
IV Establishment of Cytotoxicity by in vitro Toxicological Assay
i Brine Shrimp Lethality Assay
A stock solution of 1 mg/mL concentration was made by dissolving the test in 2% DMSO. Brine shrimp eggs (Artemia salina Leach) were allowed to hatch and mature nauplii (Larvae) in artificial sea water at 25-28ºC, under a constant light regimen. The experiment was performed by following the procedure described by Meyer [13].
Mortality (%) =
\[\frac{No.\ of\ survival\ in\ control- No.\ of\ survival\ in\ treatment}{No.\ of\ survival\ in\ control}\times100\]
ii Trypan Blue Dye Exclusion Method
Cell populations were sampled with different concentration of drugs, mixed with dye and live versus dead cells counted in Neubauer’s chamber by light microscopy [14].
\[Percentage\ viability=\frac{Number\ of\ viable\ cells\ }{Total\ number\ of\ cells\ }\times100\]
iii Micronuclei Induction
Zakerana keralenisis premetamorphic larvae (weight- 3.44 ± 1.06g and length 73.4 ± 6. 99mm, stages of 28-29) were treated with different concentrations of compounds and standard (cyclophosphamide) [15]. The micronuclei frequency was determined in 1,000 erythrocytes from each tadpole using 1,000X magnification [16].
iv Antimitotic Activity
Allium cepa base was immersed in different concentration of sample solution for 24h. Total number of roots formed, and root length were measured in each concentration. Squashed root tips were stained and observed under the microscope. The total number of cells and number of dividing cells were counted [17]. Mitotic index was calculated by using the formula:
\[Mitotic\ Index = \frac{Number\ of\ actively\ dividing\ cells}{Number\ of\ cells\ observed.}\times100\]
v Antiproliferative Assay
MTT assay, which measures mitochondrial dehydrogenase activity as a reflection of cell viability was performed using HeLa cell lines [18, 19]. Optical density was read at 540 nm using DMSO as blank in a microplate reader (ELISA SCAN, ERBA).
`% viability = \frac{OD\ of\ Test}{OD\ of\ Contro}\times100`
V In vivo Studies
i Experimental Animals
Healthy female Swiss albino mice (90-95 days old), weighing 20-25g, was acquired from Small Animal Breeding Station, Mannuthy, Kerala. Animals were preserved as per the guidelines of the Committee for the purpose of Control and Supervision of Experimental Animals [20].
ii Acute Oral Toxicity Study
Acute toxicity studies were performed as per OECD guideline 423 [21]. The animals were fasted 3-4h, and the compounds 1-(4-methoxyphenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one and 1-(4-chlorophenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one were assessed for the starting dose level. The animals were observed continuously for gross behavioural changes like body weight, changes in skin, fur, eyes, behavioural pattern and also signs of tremors, convulsion, salivation, diarrhoea, lethargy, sleep and coma every 30m for next 3h and finally death after 24h with special attention given during the first four hours and daily, thereafter for a total of 14 days.
iii Induction of Experimental Solid Tumor
Dalton’s lymphoma ascites (DLA) cell lines were obtained from Amala Cancer Research Center, Trissur, Kerala. The cells were preserved by serial Intraperitoneal (i.p) transplantation in mice. Full-grown tumor cell lines were aspirated aseptically from tumor bearing mice, washed with PBS, centrifuged and cell viability was checked. About 1×106 cells were injected subcutaneously into each new healthy female Swiss albino mouse [22, 23].
iv Experimental Design
The animals were grouped randomly into seven and each containing six animals.
i. Group I: Normal control mice received vehicle 0.25% carboxyl methylcellulose (CMC) p.o.
ii. Group II: Positive control mice were induced with DLA on day zero.
iii. Group III and Group IV: Mice induced with DLA on day zero treated with test compounds 2e (1-(4-methoxyphenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one) at a dose of 5 mg/kg and 10 mg/kg (suspended in 0.25% CMC) respectively on day 3, 5, 7, 10, 12 and 14.
iv. Group V and Group VI: Mice with DLA induced tumor on day zero treated with test compounds 2b (1-(4-chlorophenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one) at doses of 5 mg/kg and 10 mg/kg (suspended in 0.25% CMC) individually on day 3, 5, 7, 10, 12 and 14.
v. Group VII (standard): Mice induced with DLA tumor on day zero and then treated with Cisplatin (3.5 mg/kg, i.p) on day 3. On 15th-day animals were sacrificed for assessing the haematological and tumor parameters.
v Experimental Observation
The total body weight of the animals was recorded every three days throughout the duration of experiment. On day 15, all animals were sacrificed for analysing the haematological (RBC, WBC, Hb and platelets), tumor growth (tumor weight, and tumor weight and % inhibition in tumor volume), organ weights (Liver, kidney, lung and spleen) and histopathological parameters [24, 25].
vi Statistical Analysis
Statistical comparison and significance were analysed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc tests using Graph Pad Prism (Version 6.05, Graph Pad software Inc., la Jolla, CA, USA). Results are expressed as mean ± SEM, Data were analysed by one way ANOVA followed by Tukey’s multiple comparison test. *p<0.05, **p<0.01, ***p<0.001 as compared to control, # p<0.05, ##p<0.01, ### p<0.001 as compared to standard, ns- not significant.
VI RNA Extraction and Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) on p21
Tumors were excised from the animals and were used for the gene expression studies [26]. Total RNA was extracted using TRIzol® reagent (MCH 15596018, Invitrogen co, CA). The purity of the extracted RNA was determined using fluorimeter Qubit 3.1 (Life Technologies, USA). Verso One step RT-PCR kit (Thermo-scientific, USA) was used for the cDNA synthesis and amplification with the following primers [27, 28].
p21- 5’GCAGATCCACAGCGATATCC 3’(forward);
5’CAATCTGCTCACTGTCCACGG 3’(reverse)
VII Analysis of DNA Content and Cell Cycle Distribution Using Muse Cell Cycle Kit by Flow Cytometry
Cells were treated with 2e, washed and fixed with 66% ethanol at 4ºC overnight. On the next day, the cells were washed twice in PBS (PBS containing 25 mg/mL PI, 0.03% NP-40, 40 mg/mL RNAase A, at 37ºC in the dark for 30 min, (MCH100106) and labelled with PI. The study was done according to the previously reported method. The cell cycle distribution was analysed using a flow cytometer (MuseTM, Millipore, USA) [28, 29].
Results and Discussion
I In vitro Studies
In the present study, a series of chalcones [3-(3-phenoxyphenyl)-1-phenylprop-2-en-1-one (2a); 1-(4-chlorophenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one (2b); 1-(4-fluorophenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one (2c); 1-(4-Nitro-phenyl)-3-(3-phenoxy-phenyl)prop-2-en-1-one (2d); 1-(4-methoxyphenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one(2e)]were screened for in vitro cytotoxicity and micronuclei genotoxic activity. The cytotoxic potentials of chalcones were estimated using in vitro biological assays like Brine shrimp lethality assay and trypan blue dye exclusion test. Genotoxicity of the compounds were assessed by Micronuclei test and Allium cepa root tip assay.
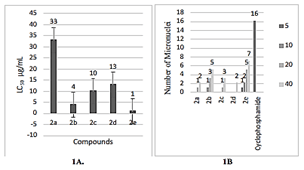
Figure 1: A)LC50 values of different chalcones using Brine shrimp lethality assay. B) Micro nuclei induction in Zakerana keralenisis tadpole on different chalcones and standard.
i Brine Shrimp Lethality Assay
The assay served as a preliminary method for the assessment of cytotoxic potential of various compounds. Brine shrimp lethality assay exhibited an increase in percentage mortality, as the concentration of test increases. Compound 2e explored 100% death at a concentration of 50 μg/mL whereas 2b showed 100% death at 100 μg/mL. The LC50 values of chalcones at 24thh were obtained from a plot of percentage mortality against different concentrations of the compounds (Figure 1A). The results obtained from brine shrimp lethality assay revealed that 2e showed maximum cytotoxicity with least LC50 value of 1μg/ml followed by 2b with LC50 value of 4μg/ml.
ii Cell Viability Assay Trypan Blue Dye Exclusion Assay
Trypan blue is an acid dye that has two azo chromophore groups. It was used in estimating the number of viable cells present in a population. This assay was used to determine the cell viability, were the dead cells get stain and appeared as dark blue in colour. The compounds showed concentration dependent cytotoxicity against DLA cells. Compound 2e exhibited maximum cell death in DLA cells with an IC50 of 48 μg/mL followed by 2b (68 μg/mL).
iii Evaluation of Micronuclei Induction
Micronuclei test is usually used in the toxicological screening to analyse DNA damage. It is well-known that DNA damage induces apoptosis, one of the core mechanisms of anticancer drugs. The frequencies of micronuclei after treatment were shown (Figure 1B). A significant micronuclei induction was found in tadpoles exposed to cyclophosphamide. Tadpoles exposed to 2e showed an increase in micro nucleated erythrocytes.
iv Evaluation of Antimitotic Effect
Antimitotic evaluation was done by using Allium cepa (common onion) roots. The root tip meristems have been widely used for the evaluation of cytotoxic and anti-mitotic activity of various compounds. Allium proved as a rapid, reliable, and inexpensive system by which the antimitotic effects of various chemical compounds may monitored [30]. All the test samples inhibited root growth and mitosis when compared to the control. The mitotic index was calculated (Table 1). 2e showed potent antimitotic activity, which may be either because of a direct cytotoxic effect of the synthesized compounds or restriction of cell division in the normal cell cycle.
Table 1: Number of roots, root length and mitotic index in Allium cepa on various chalcones,
control and standard.
|
Conc in
µg/mL |
Root
number, Root length and mitotic index after 24 hours ( Mean ± SD) |
|||||
|
2b |
2e |
|||||
|
No. of
roots |
Root
length in cm |
MI
(%) |
No. of
roots |
Root
length in cm |
MI
(%) |
|
|
0.2 |
5.66± 0.57 |
0.71± 0.03 |
72 |
4.66± 0.57 |
0.69± 0.05 |
70 |
|
0.4 |
4.33± 0.47 |
0.51± 0.03 |
63 |
3.66± 0.55 |
0.44± 0.04 |
59 |
|
0.8 |
2.66± 0.49 |
0.41± 0.03 |
51 |
2.33± 0.51 |
0.37± 0.07 |
45 |
|
1.0 |
2.33± 0.57 |
0.24± 0.02 |
33 |
2.± 0.57 |
0.21± 0.009 |
24 |
Values
are expressed as mean ± SEM.
Based on the above data, it was found that the compound 2e and 2b showed maximum cytotoxicity and genotoxic effects. The above promising compounds were further explored to anticancer activity by using DLA induced solid tumor model.
II In vivo Studies
i Acute Toxicity Studies
OECD 423 (acute toxic class method) 2e and 2b were tested at a dose of 5 mg/kg, and subsequently with 50 and 300 mg/kg body weight. No sign of toxicity was observed for 5 and 50 mg/kg. At a dose, level (300 mg/kg) produces some signs of toxicity, including tremor, salivation, lethargy, sleep and coma. It showed signs of pain immediately after the administration of drug. One animal was found dead within 24h.
ii Antitumor Activity of 2e and 2b on DLA Induced Solid Tumor Model in Mice
Solid tumor is an abnormal mass of tissue that does not contain cyst or liquid and are mostly epithelial in nature. In the present investigation, the antitumor activity of 2e and 2b was carried out in DLA induced solid tumor model in Swiss albino mice. 2e (10 mg/kg) treated groups significantly (p<0.001) reduced the tumor volume, tumor weight and the percentage inhibition 71.25% indicated that 2e plays a direct effect on tumor cells, which enhances the curative therapy (Table 2).
Table 2: Tumor volume, tumor weight and percentage inhibition
of tumor volume of animals treated with control, different doses test and
Cisplatin.
|
Parameters |
Positive control |
2e 5mg/kg |
2e 10mg/kg |
2b 5mg/kg |
2b 10mg/kg |
Standard |
|
Tumour volume (mm3) |
0.5431± 0.1864### |
0.3465± 0.0556** |
0.2909± 0.0531*** |
0.4645± 0.1408### |
0.3983±
0.1065### * |
0.2340± 0.0319*** |
|
Tumour weight[grams] |
0.4033±
0.0176### |
0.170± 0.0208*** |
0.1267± 0.0088*** |
0.3167± 0.0240* ### |
0.2633± 0.0218**### |
0.09967± 0.0057*** |
|
% inhibition in tumour volume |
|
59.35% |
71.25% |
23.66% |
41.97% |
82.14% |
Values
are expressed as mean ± SEM.
a Effect of 2e and 2b on Body Weight
All groups showed a small decrease in body weight up to 5th day and then exhibited a slight increase in the body weight (Figure 2). A significant reduction of gain in body weight was observed in the standard treated groups.
Figure 2: Effect of 2e and 2b on the body weight of DLA induced solid tumor in mice.
b Effect of 2e and 2b on Tumor Growth Parameters in DLA Induced Solid Tumor in Mice
The reliable criteria for judging the value of any antitumor drugs includes reduction in tumor volume, tumor weight and increase in percentage inhibition. Mice treated with 2e and 2b were found significantly lowering the tumor volume when compared with the control group. The average tumor volume of the control group was found to be 0.5431±0.18 mm3. Animals treated with 2e- (5 mg/kg and 10 mg/kg) exhibited a decrease in tumor volume (0.3465±0.05 and 0.2909±0.05 mm3 respectively) which was comparable with the standard Cisplatin (0.2340±0.03193 mm3). To confirm these findings, the tumor weight was recorded after excision. A significant reduction in average tumor weight (71.25%) was found in the treated animals (2e, 10mg/kg) (Table 2), The statistical analysis showed that there is no significant difference between the standard Cisplatin (82.14%) and compound 2e at a. dose of 10mg/kg. The reduction in tumor volume and increased percentage inhibition indicated that 2e plays a direct role in killing the tumor cells and enhances the curative effect of tumor chemotherapy.
c Effect of 2e and 2b on Organ Weight of DLA Treated Mice on 15th Day
Effects of Cisplatin and chalcones on organ weights were analysed, which includes liver, kidney, lung and spleen (Table 3). The induction of solid tumor resulted from an increase in lungs, spleen and liver weight, whereas a reduction in kidney weight was also observed. On treatment with the test, exhibited significant differences (P<0.001) with their respective positive control groups and reversed all organ weights towards normal. The increase in spleen weight in the positive control group may be due to the immunological response against the host to repel the foreign tissue or the transplanted carcinoma cells.
Table 3: Effects of Cisplatin and different doses of chalcones
on various organ weights.
|
Organ |
Positive
control |
Normal |
2e 5mg/kg
|
2e 10mg/kg |
2b 5mg/kg
|
2b 10mg/kg |
Standard |
|
Liver
[gms] |
1.390±
0.0201 ### |
1.043±
0.009*** |
1.180±
0.006***### |
1.150±
0.006*** ## |
1.34±
0.015### |
1.287±
0.0120** ### |
1.060±
0.015*** |
|
Kidney
[gms] |
0.147±
0.003 ### |
0.240±
0.010 *** # |
0.183±
0.033*** ## |
0.193±
0.033*** # |
0.163±
0.033* ### |
0.173±
0.033** ### |
0.210±
0.006 *** |
|
Spleen
[gms] |
0.165±
0.003 ### |
0.10±0.001***
|
0.125±
0.003*** ## |
0.118±
0.006*** |
0.145±
0.003** ### |
0.125±
0.003*** ## |
0.105±
0.003*** |
|
Lungs
[gms] |
0.135±
0.003 ### |
0.105±
0.003 *** |
0.115±
0.003 ** |
0.108±
0.005 *** |
0.130±
0.004 ## |
0.125±
0.003 ## |
0.115±
0.003*** |
Values are expressed as mean ± SEM.
d Effect of 2e and 2b on Haematological Parameters
The results of the haematological parameters are given (Table 4). The RBC and haemoglobin levels were significantly reduced in the positive control group. Cancer may result in myelosupression and anaemia. In tumor bearing mice elevated WBC count and, reduced Hb and RBC count were observed. Anaemia occurs due to iron deficiency, either by haemolytic or myelopathic conditions, which may finally lead to reduced RBC count. The reversal of Hb content, RBC, platelets and WBC count by 2e treatment towards the normal values indicates the protective action on the hemopoietic system. In continuation, antineoplastic activity of 2e was further supported by histopathological studies.
Table 4: Effect of 2e and 2b on haematological parameters.
|
Parameters |
Positive control |
Normal |
2e 5mg/kg |
2e 10mg/kg |
2b 5mg/kg |
2b 10mg/kg |
Standard |
|
WBC (cells/mm³) |
15633± 233.3### |
7233± 145.3*** |
9933± 233.3***## |
8500± 275.4*** ns |
13900± 173.2**### |
12800± 251.7***### |
8267± 145.3*** |
|
RBC (1×10 6cells/mm³) |
2.033± 0.033### |
4.70± 0.116***## |
3.233± 0.333***### |
3.733± 0.088*** |
2.4±0.1 ### |
2.73± 0.088**### |
4.13± 0.067***
|
|
Hb (gm/dL) |
8.2± 0.173### |
15.75± 0.173*** |
13.23± 0.145***## |
13.9± 0.058 *** |
9.3± 0.416 ### |
10± 0.208**### |
14.83± 0.202*** |
|
Platelets (lack cells/mm³) |
7.333± 0.089 ### |
3.10± 0.116***## |
4.733± 0.088***### |
4.30± 0.058*** # |
6.733± 0.0889** ### |
6.567± 0.067**### |
3.733± 0.145*** |
Values are expressed as mean ± SEM.
e Histopathological Studies
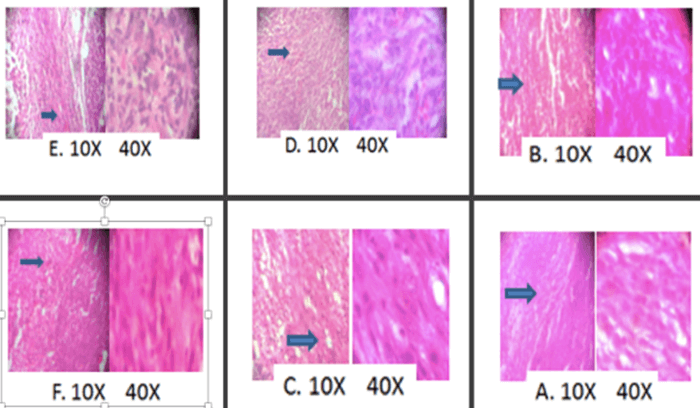
Subcutaneous tumors were processed for histological examination. Sections from the centre of each tumor were stained with haematoxylin and eosin (H and E) (Figure 3). Tumor tissue was photomicrographed at 10X and 40X magnification. Figure 3A represented H and E staining of the positive control group, show well differentiated and large area of confluent tumor cells with solid sheet like pattern morphology. No tumor tissue necrosis was observed. Figure 3B 2e-5mg/kg: treated section shows distinct necrosis like morphology, shows the presence of large vacuoles, which is an indication of necrosis. Figure 3C 2e-10mg/kg: the sections show skeletal muscle tissue with a low-grade carcinoma neoplasm. Figure 3D 2b-5mg/kg: it shows limbs tissue with infiltrating tumor composed of highly pleomorphic cancerous cells. Figure 3E 2b-10mg/kg: it shows limbs tissue with carcinoma neoplasm with minor tissue necrosis. Figure 3F standard: Cisplatin treated groups shows the fibro skeletal muscle fiber with little malignant cell infiltration. Based on the encouraging data obtained from in vivo studies, further studies for screening the mechanism behind activity of 2e was performed.
Figure 3: Histopathological analysis of control, test and standard treated albino Wistar rats on DLA-induced solid tumor model. A) Positive control. B 2e (5mg). C) 2e (10mg). D) 2b (5mg). E) 2b (10mg). F) Standard.
III Antiproliferative Study
Antiproliferative activity of the compound was assessed by MTT assay. It measures the metabolic activity of living cells by their ability to reduce the tetrazolium salt, 3- (4, 5- dimethyl thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide, to form formazan using the dehydrogenase enzymes in the mitochondria. Percentage inhibition of HeLa cells was directly proportional to the concentration of 2e and exhibited an antiproliferative effect with an IC50 value of 16μg/mL.
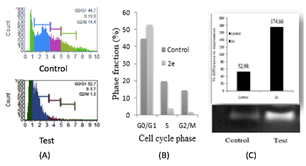
Figure 4: A) Analysis of cell cycle by flowcytometry. DNA content index histogram of control and Test (2e). B) Phase fraction in cell cycle phases. C) RTPCR analysis of p21 gene expression in HeLa cells using RT-PCR analysis.
IV DNA Content Analysis and Cell Cycle Distribution Using Muse Cell Cycle Kit by Flow Cytometry
Cell cycle analysis and gene expression studies were done to identify the targets of 2e, which revealed the DNA content proportion of G0/G1 phase increased compared to normal phase, indicating cell cycle arrest at G0/G1 phase. HeLa cells were treated with IC50 (16μg/ml) concentration of compound 2e, the percentage of cells in the G0/G1 phase increased significantly from 44.7% to 52.7%. A relative decrease in cell percentage was also observed in G2/M and S phase (Figures 4A & 4B). The results suggested the inhibitory effect of 2e on cancer cells might be due to G0/G1 arrest of the cell cycle.
V Study of Effect of 2e on p21 Gene Expression
To reveal the molecular mechanism of cell-cycle arrest in the G0/G1phase, the effect of 2e on the level of p21 was studied by gene expression analysis. Over expression of p21 after 2e treatment showed (Figure 4C). The percentage expression of 2e on p21 was found to be 174.66% and for control is only 52.98%. An important characteristic of tumor cells is their increased proliferative capability, which is often caused by impaired regulation of the cell cycle. The cell cycle has several checkpoints that are controlled by several complex modulation systems including the cyclins, cyclin-dependent kinases, and cyclin dependent kinase inhibitors. Cyclin-dependent kinase inhibitors are negative regulators of cell cycle progression and considered to be potential tumor suppressor genes. From the above results it is clear that the above chalcone (2e) have potent antitumor activity by overexpressing p21 gene. The p21 gene is a broad-acting cyclin dependent kinase inhibitor. The expression of p21 inhibits tumor growth in both in vitro and in vivo as well as induces apoptosis. The p21 gene is, therefore, an ideal target for gain-of-function manipulation to inhibit cancer cell growth and reverse tumor phenotypes [31].
Arianingrum et al. reported that chalcones significantly increased the expression of p21 and p27 proteins, and decreases the levels of cyclin B1, cyclin A and Cdc2, thereby contributing to cell cycle arrest [11]. A major target of p21 is the inhibition of cyclin E-Cdk2 complex whose activity is required for “G1” progression into “S” phase. In addition, p21 protein is able to inhibit DNA replication. p21 binds to cyclin-Cdk complexes and inhibited the activity of multiple Cdks including Cdk4 and Cdk2. The cyclin-Cdks play an important role in controlling the cell cycle transition; occur in G1 and G2-M phases [32, 33]. The possible mechanism behind 2e, it leads to the over expression of p21 in cells. The p21 gene binds to the cyclin-Cdk complexes and inhibited the activity of several cyclins dependent kinases (Cdk 2 and Cdk 4). They play an important role in the cell cycle at G0/G1phase, which may lead to the cell cycle arrest and tumor growth inhibition.
Conclusion
Chalcones constitute one of the major classes of anticancer agents which have shown promising therapeutic efficacy in the treatment of human cancers. Modifications on chalcone chromophore with hydroxyl, methoxy, and amino groups have shown promising anticancer activity. In the present study, five chalcones were screened for cell viability and DNA damage. 1-(4-methoxyphenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one (2e) and 1-(4-chlorophenyl)-3-(3-phenoxyphenyl) prop-2-en-one (2b) exhibited potent in vitro cytotoxic activity when compared with other chalcones. The former also revealed strong antiproliferative activity against HeLa cell lines. The in vivo study also confirmed antitumor activity of the methoxy derivative by reducing the tumor volume significantly and thereby increased the percentage inhibition of tumor growth and reversing the haematological parameters. Histopathological analysis also supported its anticancer activity by exhibiting low grade carcinoma neoplasm. Flow cytometry analysis showed the cell cycle arrest at G0/G1 phase. RT-PCR analysis revealed that the 1-(4-methoxyphenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one up regulated the p21 gene expression which may result in the inhibition of cyclin Cdk complex. Further research work is needed to establish the role of p21 on cyclin-Cdk complex.
Highlights of the Findings and Novelties
• Brine shrimp lethality assay, Trypan blue dye exclusion assay, Micronuclei induction, Antimitotic activity and MTT assay proved the cytotoxic activity of the chalcone derivatives
• Two promising compounds were screened for the in vivo DLA induced solid tumor model and the result showed that 1-(4- methoxyphenyl)-3-(3-phenoxyphenyl) prop-2-en-1-one can significantly reduce the tumor volume and increase the percentage inhibition.
• Histopathological analysis further confirmed the antitumor activity by showing low grade carcinoma neoplasm
• Promising chalcone induces cell cycle arrest in G0/G1 phase
• Over expression of p21 gene expression which may result in the inhibition of cyclin Cdk complex.
Ethical Approval
The experiments were carried out in accordance with the ethical norms permitted by the Institutional Animal Ethical Committee (IAEC NO: DPS/14/2015).
Acknowledgment
Our sincere thanks to Prof. N Gopalankutty and Prof. KK Srinivasan, Manipal College of Pharmaceutical Sciences, Manipal for the constant encouragement and Ms. Irine Maria Jimmy for the language correction.
Conflicts of Interest
None.
Funding
This research received funding from Kerala State Council for Science, Technology and Environment, an autonomous body of the Government of Kerala as per order No. 1210/2016/KSCSTE.
Author Contributions
The study was designed, cell culture maintenance, the in vitro, in vivo, data analysis, interpretation of results and preparation of the manuscript were performed by Dr. Litty Joseph and Lakshmi PS.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 22, Jul 2021Accepted: Wed 06, Oct 2021
Published: Mon 08, Nov 2021
Copyright
© 2023 Litty Joseph. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJCST.2021.02.04
Author Info
Corresponding Author
Litty JosephProfessor, Department of Pharmaceutical Sciences, Mahatma Gandhi University, Cheruvandoor Campus, Ettumanoor, Kottayam, Kerala, India
Figures & Tables
Table 1: Number of roots, root length and mitotic index in Allium cepa on various chalcones,
control and standard.
|
Conc in
µg/mL |
Root
number, Root length and mitotic index after 24 hours ( Mean ± SD) |
|||||
|
2b |
2e |
|||||
|
No. of
roots |
Root
length in cm |
MI
(%) |
No. of
roots |
Root
length in cm |
MI
(%) |
|
|
0.2 |
5.66± 0.57 |
0.71± 0.03 |
72 |
4.66± 0.57 |
0.69± 0.05 |
70 |
|
0.4 |
4.33± 0.47 |
0.51± 0.03 |
63 |
3.66± 0.55 |
0.44± 0.04 |
59 |
|
0.8 |
2.66± 0.49 |
0.41± 0.03 |
51 |
2.33± 0.51 |
0.37± 0.07 |
45 |
|
1.0 |
2.33± 0.57 |
0.24± 0.02 |
33 |
2.± 0.57 |
0.21± 0.009 |
24 |
Values
are expressed as mean ± SEM.
Table 2: Tumor volume, tumor weight and percentage inhibition
of tumor volume of animals treated with control, different doses test and
Cisplatin.
|
Parameters |
Positive control |
2e 5mg/kg |
2e 10mg/kg |
2b 5mg/kg |
2b 10mg/kg |
Standard |
|
Tumour volume (mm3) |
0.5431± 0.1864### |
0.3465± 0.0556** |
0.2909± 0.0531*** |
0.4645± 0.1408### |
0.3983±
0.1065### * |
0.2340± 0.0319*** |
|
Tumour weight[grams] |
0.4033±
0.0176### |
0.170± 0.0208*** |
0.1267± 0.0088*** |
0.3167± 0.0240* ### |
0.2633± 0.0218**### |
0.09967± 0.0057*** |
|
% inhibition in tumour volume |
|
59.35% |
71.25% |
23.66% |
41.97% |
82.14% |
Values
are expressed as mean ± SEM.
Table 3: Effects of Cisplatin and different doses of chalcones
on various organ weights.
|
Organ |
Positive
control |
Normal |
2e 5mg/kg
|
2e 10mg/kg |
2b 5mg/kg
|
2b 10mg/kg |
Standard |
|
Liver
[gms] |
1.390±
0.0201 ### |
1.043±
0.009*** |
1.180±
0.006***### |
1.150±
0.006*** ## |
1.34±
0.015### |
1.287±
0.0120** ### |
1.060±
0.015*** |
|
Kidney
[gms] |
0.147±
0.003 ### |
0.240±
0.010 *** # |
0.183±
0.033*** ## |
0.193±
0.033*** # |
0.163±
0.033* ### |
0.173±
0.033** ### |
0.210±
0.006 *** |
|
Spleen
[gms] |
0.165±
0.003 ### |
0.10±0.001***
|
0.125±
0.003*** ## |
0.118±
0.006*** |
0.145±
0.003** ### |
0.125±
0.003*** ## |
0.105±
0.003*** |
|
Lungs
[gms] |
0.135±
0.003 ### |
0.105±
0.003 *** |
0.115±
0.003 ** |
0.108±
0.005 *** |
0.130±
0.004 ## |
0.125±
0.003 ## |
0.115±
0.003*** |
Values are expressed as mean ± SEM.
Table 4: Effect of 2e and 2b on haematological parameters.
|
Parameters |
Positive control |
Normal |
2e 5mg/kg |
2e 10mg/kg |
2b 5mg/kg |
2b 10mg/kg |
Standard |
|
WBC (cells/mm³) |
15633± 233.3### |
7233± 145.3*** |
9933± 233.3***## |
8500± 275.4*** ns |
13900± 173.2**### |
12800± 251.7***### |
8267± 145.3*** |
|
RBC (1×10 6cells/mm³) |
2.033± 0.033### |
4.70± 0.116***## |
3.233± 0.333***### |
3.733± 0.088*** |
2.4±0.1 ### |
2.73± 0.088**### |
4.13± 0.067***
|
|
Hb (gm/dL) |
8.2± 0.173### |
15.75± 0.173*** |
13.23± 0.145***## |
13.9± 0.058 *** |
9.3± 0.416 ### |
10± 0.208**### |
14.83± 0.202*** |
|
Platelets (lack cells/mm³) |
7.333± 0.089 ### |
3.10± 0.116***## |
4.733± 0.088***### |
4.30± 0.058*** # |
6.733± 0.0889** ### |
6.567± 0.067**### |
3.733± 0.145*** |
Values are expressed as mean ± SEM.

References
1.
Jaganti V, Das S, Saisampath T (2011) A review on cancer
vaccines. Intl J Phar Bio Sci 2: 86-97.
2.
IARC
(2018) Global cancer data 2018 : IARC. Glob Cancer Obs 13-15.
3.
Weisshaar
E (2016) Cancers. Pruritus 2: 283-287.
4.
Dhillon
CFPK, Mathur P (2018) The burden of cancers and their variations across the
states of India : The Global Burden of Disease Study 1990 – 2016. Lancet
Oncol 19: 1289-1306. [Crossref]
5.
Vijayalakshmi A, Kumar PR, Priyadarsini S, Meenaxshi C (2013)
In vitro Antioxidant and Anticancer Activity of Flavonoid Fraction from
the Aerial Parts of Cissus quadrangularis linn. against Human Breast
Carcinoma Cell Lines. J Chem 1-8.
6.
Kumar N, Dhamija I, Vasanth Raj P, Jayashree BS, Parihar V et
al. (2014) Preliminary investigation of cytotoxic potential of 2-quinolone
derivatives using in vitro and in vivo (solid tumor and liquid
tumor) models of cancer. Ara J Chem 7: 409-417.
7.
Singh P, Anand A, Kumar V (2014)
Recent developments in biological activities of chalcones : A mini review. Eur
J Med Chem 85: 758-777. [Crossref]
8. Venturelli
S, Burkard M, Biendl M, Lauer UM, Frank J et al. (2016) Prenylated chalcones
and flavonoids for the prevention and treatment of cancer. Nutrition 32:
1171-1178. [Crossref]
9. Marković
V, Debeljak N, Stanojković T, Kolundžija B, Sladić D (2015)
Anthraquinone-chalcone hybrids : synthesis, preliminary antiproliferative
evaluation and DNA-interaction studies. EurJ Med Chem 89: 401-410. [Crossref]
10.
Sankappa Rai U, Isloor AM, Shetty P,
Pai KSR, Fun HK (2015) Synthesis and in vitro biological evaluation of
new pyrazole chalcones and heterocyclic diamides as potential anticancer
agents. Ara J Chem 8: 317-321.
11. Arianingrum
R, Sunarminingsih R, Meiyanto E, Mubarika S (2015) Synergistic effects of para-
hydroxy meta- methoxy chalcone (pHmMC) - doxorubicin treatments on T47D breast
cancer cells. Indo J Biot 20: 141-151.
12. Susanti VH E, Setyowati WAE (2018)
Green Synthesis of Chalcones As an Antioxidant and Anticancer. IOP Conf Ser
Mater Sci Eng 299 (2017): 012077.
13. Meyer
BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE et al. (1982) Brine shrimp:
a convenient general bioassay for active plant constituents. Planta Med 45:
31-34. [Crossref]
14. Sunila ES, Kuttan G, KC Preethi,
Kuttan R (2007) Effect of homeopathic medicines on transplanted
tumors in mice. Asian Pac J Cancer Prev 8: 390-394. [Crossref]
15.
Gosner KL (1960) A Simplified Table
for Staging Anuran Embryos and Larvae with Notes on Identification. Herpetologica
16:183-190.
16.
Campana MA, Panzeri AM, Moreno VJ,
Dulout FN (2003) Micronuclei induction in Rana catesbeiana tadpoles by the
pyrethroid insecticide lambda-cyhalothrin. Gen Mol Bio 26: 99-103.
17. Auti
S, Pagare R, Ahire D, Sawale V (2010) Cytogenetical studies on the effect of
omnacortil on root tip cells of Allium cepa L. J Cell Tiss Res 10:
2331-2335.
18. Mosmann
T (1983) Rapid colorimetric assay for cellular growth and survival: application
to proliferation and cytotoxicity assays. J Immunol Methods 65: 55-63. [Crossref]
19. Atta
ur Rahman, ChoudharyMI, Thomsen WJ (2005) Bioassay Techniques for Drug
Development. Hard Acad Publ.
20. CPCSEA
(2018) Compendium of CPCSEA. Mini Envir Fore Clim Chan Gov Ind.
21.
OECD (2002) Test No. 423: Acute Oral
toxicity - Acute Toxic Class Method. OECD Guid Test Chemi 1-14.
22. Kuttan
G, Vasudevan DM, Kuttan R (1990) Effect of a preparation from Viscum album on
tumor development in vitro and in mice. J Ethanopharmacol 29: 35-41. [Crossref]
23.
Joseph L, Aranjani JM, Pai KSR,
Srinivasan KK (2017) Promising anticancer activities of Justicia simplex D.
Don. in cellular and animal models. J Ethnopharmacol 199: 231-239. [Crossref]
24. Raghavendra
NM, Gurubasavarajaswamy PM, Nagaranavile KS, Parameshwaran T (2009) Antitumor
actions of imidazolyl-(4-oxoquinazolin-3(4H)-yl)-acetamides against Ehrlich
Ascites Carcinoma. Arch Pharm Res 32: 431-436. [Crossref]
25. Anand
K, Asthana P, Kumar A, Ambasta RK, Kumar P (2011) Quercetin mediated reduction
of angiogenic markers and chaperones in DLA-induced solid tumours. Asian Pac
J Cancer Prev 12: 2829-2835. [Crossref]
26. Anthony
M (2010) Effect of H2O2 on Adult Neuralstem/Progenitor Cells. J Neuro
228: 211-219.
27. Xia
G, Han X, Qi J, Liu W, Song J et al. (2012) The effects of Astragalus
polysaccharide on zebrafish cell apoptosis and senescence. Am J Molr Bio
2: 103-109.
28. Joseph
L, Srinivasan KK (2019) Triacontanoic ester of 5"-hydroxyjustisolin:
Tumour suppressive role in cervical cancer via Bcl-2, BAX and caspase-3
mediated signalling. Toxicol Rep 6: 1198-1205. [Crossref]
29. Bhat
TA, Nambiar D, Pal A, Agarwal R, Singh RP (2012) Fisetin inhibits various
attributes of angiogenesis in vitro and in vivo--implications for
angioprevention. Carcinogenesis 33: 385-393. [Crossref]
30. Shachi
S (2012) Antimitotic activity of a New Compound Isolated from the Flower of
Prosopis juliflora. Res J Rece Tim 1: 22-26.
31. Chen
X, Xu H, Yuan P, Fang F, Huss M et al. (2008) Integration of external signaling
pathways with the core transcriptional network in embryonic stem cells. Cell
133: 1106-1117. [Crossref]
32. Zhang W, Grasso L, Mcclain CD, Gambel AM, Cha Y et al. (1995) p53-independent induction of WAF1/CIP1 in human leukemia cells is correlated with growth arrest accompanying monocyte/macrophage differentiation. Cancer Res 55: 668-674. [Crossref]
33. Rezaee MA, Mohammadpour AH, Imenshahidi M, Mahmoudi M, Sankian M et al. (2016) Protective effect of erythropoietin on myocardial apoptosis in rats exposed to carbon monoxide. Life Sci 148: 118-124. [Crossref]
