Prevalence and Outcome of Advanced Left Ventricular Diastolic Dysfunction among Consecutive Patients Referred for Echocardiography
A B S T R A C T
Aim: To assess the prevalence of advanced left ventricular diastolic dysfunction (LVDD) in a cohort of consecutive patients referred for echocardiography and its association with mortality.
Methods: The cohort included 4,481 (85% hospitalized) patients who underwent echocardiography, had normal or preserved LV systolic function and diastolic function assessment. LVDD was graded as none or mild (0/I) and advanced grade (II/III). Mortality data were derived from the National Israeli Population Registry.
Results: LVDD grade II/III was found in 1,262 patients (28%), was more prevalent among the elderly, females, diabetic and hypertensive patients. Independent predictors associated with LVDD grade II/III (OR; 95% CI) were: age (1-year increment) 1.015 (1.01-1.02), p<0.001; female sex 1.2 (1.04-1.39), p=0.012; hypertension 1.53 (1.30-1.80), p<0.001, while ischaemic heart disease was negatively associated 0.73 (0.63-0.85), p<0.001. 1-year mortality rates were higher among grade II/III LVDD as compared to grade 0/I DD patients, 19% vs. 10.2%, respectively, p<0.0001. Independent predictors for all-cause mortality after adjusting for pertinent variables were: LVDD grade II/III 1.72 (1.40-2.11); age (1-year increment) 1.08 (1.07-1.09) and diabetes 1.54 (1.26-1.70), p<0.001 for all.
Conclusion: LVDD grade II/III was more prevalent among the elderly, females, diabetic and hypertensive patients. Advanced LVDD was a strong independent predictor for all-cause mortality after adjustment for risk factors. Intensive pharmacological therapies at an earlier stage of LVDD may improve patients’ outcome.
Keywords
Left ventricle, diastolic dysfunction, prevalence, mortality, outcome
Introduction
Left ventricular diastolic dysfunction (LVDD) is associated with the development of heart failure with preserved ejection fraction (HFpEF), atrial fibrillation, and increased risk of cardiovascular (CV) death [1-6]. LVDD is more prevalent among the elderly, females, diabetic and hypertensive patients [6-8]. However, the relative impact of each of these risk factors on the development of LVDD is not clear. Furthermore, although LVDD is associated with an increased risk of CV death, it is not clear whether LVDD itself contributes to an additional mortality risk in the presence of CV risk factors.
The aim of the study was to evaluate in a large cohort of mostly hospitalized consecutive patients referred for echocardiography, the prevalence of advanced LVDD, and to assess its prognostic significance on mortality.
Methods
The study population included 4,481 consecutive patients, 85% hospitalized in cardiology and internal medicine wards, and others who were referred for trans-thoracic echocardiography (TTE) at our tertiary care center, from November 1, 1991, to October 31, 2017. Patients with normal or preserved left ventricular systolic function were included in the study. Patients with mitral stenosis were excluded. All patients had Doppler data that enabled the diastolic function assessment.
To evaluate the diastolic function, trans-mitral inflow was recorded using pulsed-wave Doppler in the apical 4 chamber view for measurements of early (E) and late (A) mitral inflow velocities. Early diastolic velocity was assessed at the septal (septal e’ (and lateral (lateral e’) sites of the mitral annulus using pulsed-wave tissue Doppler imaging. The average value of septal e’ and lateral e’ was used to calculate E/e’ ratio. The maximal left atrial (LA) area was measured by planimetry on apical 4-chamber view, and the LA volume by using modified Simpson’s rule in apical 4-chamber and apical 2-chamber views at ventricular end-systole. Diastolic function was evaluated according to the 2016 recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging (ASE/EACVI) [9].
In brief, in patients with normal or preserved left ventricular function, four recommended variables and their abnormal cutoff values were used: i) septal e′ < 7 cm/sec or lateral e′< 10 cm/sec; ii) average E/e′ ratio > 14; iii) peak TR velocity >2.8 m/sec; iv) LA volume index >34mL/m2. LVDD was identified when at least 2 out of these 4 variables met the cutoff values. The severity of LVDD was defined according to the following criteria: i) Normal diastolic function (Grade 0); ii) grade I when E/A <0.8 and E ≤50 cm/s; iii) Grade II when E/A <0.8 and E >50 cm/s or E/A >0.8 <2 and two of the above mentioned variables B, C or D met the cutoff points) iv) Grade III when E/A >2. LVDD grade II/III was defined as advanced LVDD. All patients’ data including demographics, CV risk factors, and echo-Doppler measurements were prospectively collected and stored in the hospital computerized patients charts database. Mortality data were derived and confirmed by the National Israeli Population Registry of the Ministry of Interior.
Statistical Analyses
Patients’ characteristics were described by means ± SD for continuous variables and numbers with percentages for categorical variables. The association between categorical variables and LVDD grade II/III were assessed using chi-square test, and the relation between continuous variables and LVDD grade II/III were tested using the unpaired Student’s t-test. Multivariable logistic regression model was conducted in order to identify independent variables associated with advanced LVDD. The variables that were included in the analysis were: age, sex, diabetes, hypertension, history of ischaemic heart disease (IHD), moderate and severe valvular diseases including aortic stenosis and regurgitation and mitral regurgitation, and patients’ status during the performance of the echocardiography study (ambulatory versus hospitalized). Odds ratios (ORs) with 95% confidence intervals (CIs) are reported.
The role of LVDD grade II/III on survival time, evaluated from the index echocardiography date are shown by Kaplan-Meier curves and were compared with the use of a log-rank test. In order to assess variables associated with 1-year all-cause mortality, Cox proportional-hazards regression model was obtained with hazard ratios (HRs) and 95% confidence intervals (CI) and included the same parameters that were contained in the analysis performed to identify the independent variables associated with advanced LVDD, including the variable LVDD grade II/III. All tests were two-sided. P-value of <0.05 was considered statistically significant. Analyses were performed by using SPSS Statistics software, version 25, IBM, Armonk, NY.
Results
The patients’ characteristics are presented in (Table 1). It is notable that the patients with LVDD grade II/III were older, more frequently females, had a higher prevalence of diabetes and hypertension, but a lower prevalence of IHD. LVDD grade II/III was more frequent among patients with moderate and severe valvular disease and hospitalized patients. Patients with LVDD grade II/III had lower fractional shortening, left atrial diameter, and Doppler parameters that reflect diastolic dysfunction. The patients’ characteristics according to the individual LV diastolic function parameters are detailed in (Table 2). It is notable that in general, these parameters are more frequent with advanced age, among females, diabetic and hypertensive patients, while they were less frequent among patients with IHD. Multivariate logistic regression analysis revealed that the variables independently associated with LVDD grade II/III were: older age, female sex, hypertension, moderate and severe valvular disease, and hospitalization status, whereas diabetes was not. The presence of IHD was associated with a lower risk of LVDD (Table 3A). When 1,204 patients with moderate and severe valvular disease were excluded from the model, female sex was no longer associated with LVDD grade II/III, whereas diabetes became positive (Table 3B).
Table 1: Clinical and Echo-Doppler characteristics of patients with grade 0/I and II/III LVDD.
|
p-Value |
LVDD grade II/III n=1,262 |
LVDD grade 0/I n=3,219 |
|
|
|
|
|
Clinical characteristics |
|
>0.001 |
76±13 |
71±13 |
Age (m±SD), years |
|
<0.001 |
719 (57) |
1,513 (47) |
Female sex (n,%) |
|
0.028 |
379 (30) |
869 (27) |
Diabetes (n,%) |
|
>0.001 |
959 (76) |
1,996 (62) |
Hypertension (n,%) |
|
<0.001 |
404 (32) |
1,223(38) |
Ischaemic heart disease (n,%) |
|
<0.0001 |
658 (52) |
546 (17) |
Moderate /Severe valvular disease *(n,%) |
|
<0.0001 |
1140 (90) |
2688( 84) |
Hospitalized patients (n,%) |
|
|
|
|
Echo-Doppler characteristics |
|
0.820 |
4.7 [4.3-4.1] |
4.8 [4.4-5.1 |
Left Ventricular End Diastolic diameter (cm) |
|
0.015 |
2.9 [2.6-3.3] |
2.9 [2.6-3.2] |
Left Ventricular End Sytolic diameter (cm) |
|
<0.001 |
38 [33-43] |
39 [35-44] |
Fractional shortening % |
|
<0.0001 |
4.8 [4.4-5.2] |
4.2 [3.8-4.7] |
Left Atrial diameter (short axis) cm |
|
0.007 |
6.1 [5.0-7.5] |
6.4 [5.2-8.0] |
e' septum velocity cm/s |
|
0.168 |
8.2 [6.8-10.1] |
8.4 [6.9-10.2] |
e' lateral velocity cm/s |
|
<0.0001 |
107 [89-125] |
76 [62-93] |
E velocity cm/s |
|
<0.0001 |
47 [38-67] |
78.3 [64-94] |
A velocity cm/s |
|
<0.0001 |
2.23 [1.73-2.73] |
0.91 [0.73-1.24] |
E/A |
|
<0.0001 |
659 (52.2) |
0 |
E/A >2 |
|
<0.0001 |
14.7 [11.68-18.52] |
10 [7.83-12.823] |
E/e' ratio |
|
<0.001 |
310 (25) |
571 (18) |
E/e'>14 (n, %) |
|
<0.001 |
1002 (83) |
930 (29) |
TR velocity>2.8 m/s (n,%) |
|
<0.001 |
672 (97) |
282 (49) |
Left Atrial volume index ml/m2 >34 (n,%) |
*Aortic stenosis, aortic regurgitation, mitral regurgitation
Table 2: Patients' characteristics by individual LV diastolic function parameters.*
|
|
E/A |
E/e' |
TR velocity m/s |
Left Atrial volume index ml/m2 |
||||||||
|
|
<2 |
>2 |
p-Value |
<14 |
>14 |
p-Value |
<2.8
|
>2.8
|
p-Value |
<34 |
>34 |
p-Value |
|
n |
3,822 |
659 |
|
3,600 |
881 |
|
2,478 |
1,932 |
|
319 |
954 |
|
|
Age (m±SD) years" |
72.39±13 |
72.9±14 |
0.358 |
70.9±13 |
78.7±10 |
<0.001 |
68.5±13 |
77.9±11 |
<0.001 |
70.4±12 |
77.4±11 |
<0.001 |
|
Female (%) |
50.2 |
47.5 |
0.203 |
46.2 |
64.4 |
<0.001 |
42.3 |
60.4 |
<0.001 |
49.2 |
62.1 |
<0.001 |
|
Diabetes (%) |
27.3 |
31.4 |
0.030 |
25.1 |
39.6 |
<0.001 |
24.2 |
32.1 |
<0.001 |
25.7 |
27.1 |
0.614 |
|
Hypertension (%) |
65.8 |
69.2 |
0.084 |
62.8 |
80.5 |
<0.001 |
56.5 |
79.2 |
<0.001 |
62.7 |
78.5 |
<0.001 |
|
Ischaemic heart disease (%) |
35.6 |
37.6 |
0.030 |
36.1 |
35.2 |
0.630 |
39.1 |
31.6 |
<0.001 |
38.2 |
27.0 |
<0.001 |
*Diastolic function assessment, 2016 ASE/EACVI [9].
Table 3: Independent predictors for LVDD grade II/III.*
|
|
OR* |
95% CI |
p-Value |
|
A. All patients |
|
|
|
|
Age (1-year increment) |
1.015 |
1.01-1.02 |
<0.001 |
|
Female sex |
1.20 |
1.04-1.39 |
0.012 |
|
Hypertension |
1.53 |
1.30-1.80 |
<0.001 |
|
Diabetes |
1.16 |
0.99-1.35 |
0.067 |
|
Ischaemic heart disease |
0.73 |
0.63-0.85 |
<0.001 |
|
Moderate/Severe valvular diseaseᵻ |
4.88 |
4.00-5.94 |
<0.001 |
|
Hospitalized patients |
1.93 |
1.55-2.42 |
<0.001 |
|
B. Valvular disease patients excludedᵻ: |
|
|
|
|
Age (1-year increment) |
1.011 |
1.003-1.020 |
0.006 |
|
Female sex |
0.98 |
0.81-1.18 |
0.83 |
|
Hypertension |
1.55 |
1.26-1.92 |
<0.0001 |
|
Diabetes |
1.29 |
1.06-1.57 |
0.012 |
|
Ischaemic heart disease |
0.61 |
0.50-0.75 |
<0.0001 |
*By logistic regression analysis (see Methods)
ᵻModerate and severe aortic stenosis, aortic regurgitation, mitral regurgitation
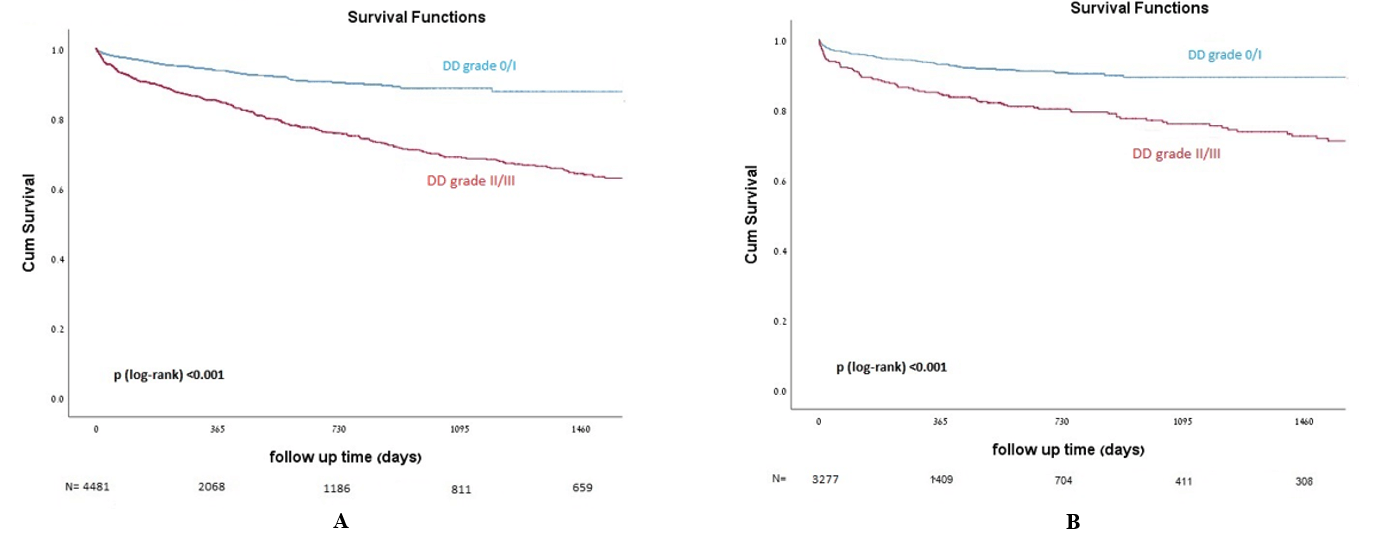
The median follow-up time was 304 (107-636) days. 1-year all-cause mortality was higher among patients with LVDD grade II/III as compared to patients with grade 0/I, 19% vs. 10.2%, respectively, p<0.0001 (Figure 1A). After excluding all patients with moderate and severe valvular (see methods), 1-year mortality rate was 16% among LVDD grade II/III as compared to 7% among LVDD grade 0/I; p<0.0001 (Figure 1B).
Figure 1: A) Kaplan-Meier cumulative survival curves of the entire cohort, comparing LVDD grade 0/I and LVDD grade II/III. B) Kaplan-Meier cumulative survival curves of the cohort excluding patients with moderate and severe valvular disease, comparing LVDD grade 0/I and LVDD grade II/III.
Cox proportional hazard regression analysis revealed that LVDD grade II/III was independently associated with increased all-cause mortality as compared to grade 0/I LVDD (HR=1.72 (1.40-2.11), p<0.001). This result was also maintained after excluding from the model all patients with moderate and severe valvular disease HR=2.08 95%CI [1.53-2.83] p<0.0001 (Table 4).
Table 4: Independent predictors of 1-year all-cause mortality.*
|
|
HR* |
95% CI |
p-Value |
|
Grade II/III diastolic dysfunction |
1.72 |
1.40-2.11 |
<0.001 |
|
Diabetes |
1.54 |
1.26-1.7 |
<0.001 |
|
Age (1-year increment) |
1.08 |
1.07-1.09 |
<0.001 |
|
Moderate /Severe valvular diseaseᵻ |
1.93 |
1.57-2.36 |
<0.001 |
|
Hospitalized patients |
1.59 |
1.15-2.21 |
0.005 |
*By Cox proportional hazards-regression model (HR) (see Methods)
ᵻModerate and severe aortic stenosis, aortic regurgitation, mitral regurgitation
Discussion
Our study included 4,481 consecutive patients (85% hospitalized) referred for echocardiography who had normal or preserved LV systolic function and diastolic function assessment. Of them, 72% had a normal diastolic function or mild dysfunction (grade I), whereas 28% had advanced LVDD grade II/III. The study demonstrated that advanced LVDD was more common among the elderly, female sex, diabetic, hypertensive, moderate and severe valvular disease, and hospitalized patients, whereas less frequent among patients with known IHD. The study demonstrated that advanced LVDD was a strong independent predictor of all-cause mortality.
Prior studies have described the association between CV risk factors and the development of LVDD; however, these studies used non-standardized methods of measurements and definitions for the diagnosis of LVDD [6-8]. Almeida et al., emphasized that the heterogeneity and ambiguity of the different definitions of LVDD led to a significant variability in its reported prevalence and grading. In their study following the release of 2016 ASE/EACVI diastolic function assessment algorithm, a retrospective analysis of a population-based cohort of 1,000 individuals was performed [9, 10]. After exclusion of those with previously known cardiac disease or ejection fraction <50%, the prevalence of LVDD was 1.4% with the 2016 recommendations, 38.1% with the 2009 recommendations, and 30.4% using the Canberra Study Criteria. Hence, they concluded that the application of the new recommendations resulted in a much lower prevalence of LVDD and that the concordance between the classifications was poor. In a recent study by Gopalakrishnan et al., that also followed the 2016 ASE/EACVI recommendations and was based on more than 20,000 echocardiograms from a single-center, it was demonstrated that there was a 57% decrease in reporting of diastolic dysfunction (p<0.001), grade 1 LVDD decreased by 64% (P < 0.001), grade 2 LVDD decreased by 51% (P <0.001), and grade 3 LVDD did not change significantly (P =0.18) [11]. Hence, diastolic function studies published before the release of the 2016 recommendations present miscellaneous non-standardized results. The high prevalence of advanced LVDD observed in our cohort using the 2016 ASE/EACVI recommendations reflects the difference between our study population and the aforementioned ones. In our cohort, most of the patients were hospitalized, and those with significant valvular disease were not excluded from the analyses.
Our study demonstrated that advanced LVDD was more common among the elderly, female sex, hypertensive, and diabetic patients, similarly to Nayor et al., [6]. In an additional article, they concluded that when age- and sex-specific reference limits are used, LVDD was less dependent on age, correlated more powerfully to systolic and diastolic blood pressure, body mass index, hypertension treatment status, total cholesterol/HDL-cholesterol and diabetes [12].
In our study, the presence of IHD was associated with a lower frequency of advanced LVDD. In contrast, in a study by Lin et al., the extent and severity of obstructive as well as non-obstructive coronary disease by coronary CT angiography were associated with increased measures of LVDD [13]. The prevalence of LVDD increased with a greater number of obstructed vessels. A possible explanation for this contrast is that the information regarding the history of IHD in our study was retrieved from the patients’ echo database and was not confirmed angiographically in all patients; hence, it might underestimate the true prevalence of coronary disease in our population. A second possible explanation for our finding may be related to the wide range of medications given to patients with IHD, including beta-blockers, ACEI and ARBS, calcium channel blockers, and nitrates that may actually reduce the risk for the development of advanced LVDD.
The impact of medications on LVDD has not been resolved yet. Several studies have shown that inhibition of the renin-angiotensin aldosterone system (RAAS) with ACEIs or ARBs improve LVDD, also with a combination with beta-blockers or calcium channel blockers; however, others failed to demonstrate such benefit, like the Valsartan in Diastolic Dysfunction (VALIDD) and the perindopril indapamide combination [14-19]. Lan et al., demonstrated that LV mass index and diastolic function improved following SGLT2 inhibitor therapy in people with type 2 diabetes [20]. Our study is retrospective, derived from the departmental electronic database, and hence, the impact of medications on diastolic function was beyond its scope.
Left Ventricular Diastolic Dysfunction (LVDD) and Outcome
In our study, 1-year all-cause mortality rates were 19% among patients with advanced LVDD (grade II/III) as compared to 10.2% among patients with grade 0/I, p<0.0001. The relatively high mortality rates observed in our patient population might be related to the fact that the majority of the patients in both groups were hospitalized and hence were sicker as compared to echo-laboratories that serve mostly outpatients.
Our study has demonstrated that echocardiographic and tissue Doppler imaging (TDI) parameters that assess diastolic dysfunction predict patients’ outcome. In accordance with our study, several prior studies have noted that moderate and severe LVDD is associated with increased mortality risk [6, 21, 22]. Mogelvank et al., in their community-based Copenhagen City Heart Study, demonstrated that left ventricular systolic and/or diastolic dysfunction assessed by TDI on 1,036 participants were independent predictors of death in the general population [21]. TDI information had an incremental predictive value when age, body mass index, heart rate, hypertension, diabetes, IHD, and plasma pro-BNP were taken into consideration. Kuznetsova et al., studied the prognostic role of TDI-derived indexes on 793 patients of a general population [22]. After adjustment for conventional cardiovascular risk factors, LV TDI mitral annular e’ velocity was a significant predictor of fatal and nonfatal cardiovascular events, as compared with a model including only conventional cardiovascular risk factors. However, mitral annular e’ velocity is just one of the parameters required for the evaluation of LVDD. We have demonstrated that advanced LVDD was a strong independent predictor for all-cause mortality after adjustment for age, sex, diabetes, hypertension, and other pertinent variables.
The ASCOT study, which included 980 patients with hypertension but without coronary heart disease demonstrated that E/e’ ratio was the strongest predictor of cardiac events by Cox-proportional hazards models, corrected for age, gender, diabetes and systolic blood pressure, and that a unit rise in the E/e’ ratio was associated with a 17% increment in the risk of a cardiac events [23]. Blomstrand et al., demonstrated that among 406 middle-aged diabetic patients that E/e′ ratio was a strong predictor of myocardial infarction and stroke comparable with HbA1c, superior to global left ventricular longitudinal strain and left ventricular ejection fraction [24]. In patients with diabetes, the E/e′ ratio has appeared to independently predict heart failure and mortality [25].
The PARAGON-HF trial tested the efficacy of sacubitril-valsartan in patients with heart failure with preserved ejection fraction (HFpEF). Among the 868 participants who had with TDI data, 96% had 2016 ASE/EACVI criteria for HFpEF. During a median follow-up of 2.8 years, 288 patients experienced heart failure hospitalization or cardiovascular death. Multiple Doppler-based diastolic measures were robustly associated with these outcomes, including higher E-wave velocity, lower TDI septal e’, and higher septal and lateral E/e’ ratios were each associated with higher risk for the composite endpoint in adjusted models [26].
Limitations
Our cohort represents mainly hospitalized patients hence reflecting a highly selected population. It is possible that our results may not be applicable to echo Doppler findings in outpatient population.
Conclusion
Utilizing the 2016 ASE/EACVI criteria for the assessment of LVDD revealed high prevalence of advanced LVDD in a cohort of mainly hospitalized patients. Advanced LVDD is more prevalent among the elderly, females, diabetic and hypertensive patients, and provides an independent powerful predictor of all-cause mortality. Patients with earlier stages of LVDD may require intensive medical attention and pharmacological therapies in an attempt to improve their outcome.
Conflicts of Interest
None.
Author Contributions
The research concept and design were outlined by MM and SG. Data acquisition, analysis and interpretation were performed by MM, SG, RF and AB. The manuscript was drafted by MM and SG. All authors contributed to critical revision and approved the final version.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 18, Dec 2020Accepted: Sat 02, Jan 2021
Published: Thu 14, Jan 2021
Copyright
© 2023 Mady Moriel. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2021.01.06
Author Info
Mady Moriel Adi Butnaru Marc Klutstein Rivka Farkash Michael Glikson David Rosenmann Shmuel Gottlieb
Corresponding Author
Mady MorielThe Jesselson Integrated Heart Center, Shaare Zedek Medical Center, Jerusalem, Israel
Figures & Tables
Table 1: Clinical and Echo-Doppler characteristics of patients with grade 0/I and II/III LVDD.
|
p-Value |
LVDD grade II/III n=1,262 |
LVDD grade 0/I n=3,219 |
|
|
|
|
|
Clinical characteristics |
|
>0.001 |
76±13 |
71±13 |
Age (m±SD), years |
|
<0.001 |
719 (57) |
1,513 (47) |
Female sex (n,%) |
|
0.028 |
379 (30) |
869 (27) |
Diabetes (n,%) |
|
>0.001 |
959 (76) |
1,996 (62) |
Hypertension (n,%) |
|
<0.001 |
404 (32) |
1,223(38) |
Ischaemic heart disease (n,%) |
|
<0.0001 |
658 (52) |
546 (17) |
Moderate /Severe valvular disease *(n,%) |
|
<0.0001 |
1140 (90) |
2688( 84) |
Hospitalized patients (n,%) |
|
|
|
|
Echo-Doppler characteristics |
|
0.820 |
4.7 [4.3-4.1] |
4.8 [4.4-5.1 |
Left Ventricular End Diastolic diameter (cm) |
|
0.015 |
2.9 [2.6-3.3] |
2.9 [2.6-3.2] |
Left Ventricular End Sytolic diameter (cm) |
|
<0.001 |
38 [33-43] |
39 [35-44] |
Fractional shortening % |
|
<0.0001 |
4.8 [4.4-5.2] |
4.2 [3.8-4.7] |
Left Atrial diameter (short axis) cm |
|
0.007 |
6.1 [5.0-7.5] |
6.4 [5.2-8.0] |
e' septum velocity cm/s |
|
0.168 |
8.2 [6.8-10.1] |
8.4 [6.9-10.2] |
e' lateral velocity cm/s |
|
<0.0001 |
107 [89-125] |
76 [62-93] |
E velocity cm/s |
|
<0.0001 |
47 [38-67] |
78.3 [64-94] |
A velocity cm/s |
|
<0.0001 |
2.23 [1.73-2.73] |
0.91 [0.73-1.24] |
E/A |
|
<0.0001 |
659 (52.2) |
0 |
E/A >2 |
|
<0.0001 |
14.7 [11.68-18.52] |
10 [7.83-12.823] |
E/e' ratio |
|
<0.001 |
310 (25) |
571 (18) |
E/e'>14 (n, %) |
|
<0.001 |
1002 (83) |
930 (29) |
TR velocity>2.8 m/s (n,%) |
|
<0.001 |
672 (97) |
282 (49) |
Left Atrial volume index ml/m2 >34 (n,%) |
*Aortic stenosis, aortic regurgitation, mitral regurgitation
Table 2: Patients' characteristics by individual LV diastolic function parameters.*
|
|
E/A |
E/e' |
TR velocity m/s |
Left Atrial volume index ml/m2 |
||||||||
|
|
<2 |
>2 |
p-Value |
<14 |
>14 |
p-Value |
<2.8
|
>2.8
|
p-Value |
<34 |
>34 |
p-Value |
|
n |
3,822 |
659 |
|
3,600 |
881 |
|
2,478 |
1,932 |
|
319 |
954 |
|
|
Age (m±SD) years" |
72.39±13 |
72.9±14 |
0.358 |
70.9±13 |
78.7±10 |
<0.001 |
68.5±13 |
77.9±11 |
<0.001 |
70.4±12 |
77.4±11 |
<0.001 |
|
Female (%) |
50.2 |
47.5 |
0.203 |
46.2 |
64.4 |
<0.001 |
42.3 |
60.4 |
<0.001 |
49.2 |
62.1 |
<0.001 |
|
Diabetes (%) |
27.3 |
31.4 |
0.030 |
25.1 |
39.6 |
<0.001 |
24.2 |
32.1 |
<0.001 |
25.7 |
27.1 |
0.614 |
|
Hypertension (%) |
65.8 |
69.2 |
0.084 |
62.8 |
80.5 |
<0.001 |
56.5 |
79.2 |
<0.001 |
62.7 |
78.5 |
<0.001 |
|
Ischaemic heart disease (%) |
35.6 |
37.6 |
0.030 |
36.1 |
35.2 |
0.630 |
39.1 |
31.6 |
<0.001 |
38.2 |
27.0 |
<0.001 |
*Diastolic function assessment, 2016 ASE/EACVI [9].
Table 3: Independent predictors for LVDD grade II/III.*
|
|
OR* |
95% CI |
p-Value |
|
A. All patients |
|
|
|
|
Age (1-year increment) |
1.015 |
1.01-1.02 |
<0.001 |
|
Female sex |
1.20 |
1.04-1.39 |
0.012 |
|
Hypertension |
1.53 |
1.30-1.80 |
<0.001 |
|
Diabetes |
1.16 |
0.99-1.35 |
0.067 |
|
Ischaemic heart disease |
0.73 |
0.63-0.85 |
<0.001 |
|
Moderate/Severe valvular diseaseᵻ |
4.88 |
4.00-5.94 |
<0.001 |
|
Hospitalized patients |
1.93 |
1.55-2.42 |
<0.001 |
|
B. Valvular disease patients excludedᵻ: |
|
|
|
|
Age (1-year increment) |
1.011 |
1.003-1.020 |
0.006 |
|
Female sex |
0.98 |
0.81-1.18 |
0.83 |
|
Hypertension |
1.55 |
1.26-1.92 |
<0.0001 |
|
Diabetes |
1.29 |
1.06-1.57 |
0.012 |
|
Ischaemic heart disease |
0.61 |
0.50-0.75 |
<0.0001 |
*By logistic regression analysis (see Methods)
ᵻModerate and severe aortic stenosis, aortic regurgitation, mitral regurgitation
Table 4: Independent predictors of 1-year all-cause mortality.*
|
|
HR* |
95% CI |
p-Value |
|
Grade II/III diastolic dysfunction |
1.72 |
1.40-2.11 |
<0.001 |
|
Diabetes |
1.54 |
1.26-1.7 |
<0.001 |
|
Age (1-year increment) |
1.08 |
1.07-1.09 |
<0.001 |
|
Moderate /Severe valvular diseaseᵻ |
1.93 |
1.57-2.36 |
<0.001 |
|
Hospitalized patients |
1.59 |
1.15-2.21 |
0.005 |
*By Cox proportional hazards-regression model (HR) (see Methods)
ᵻModerate and severe aortic stenosis, aortic regurgitation, mitral regurgitation
References
- Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR et al. (2003) Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 289: 194-202. [Crossref]
- Kane GC, Karon L, Mahoney DW, Redfield MM, Roger VL et al. (2011) Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 306: 856-886. [Crossref]
- Rosenberg MA, Gottdiener JS, Heckbert SR, Mukamal KJ (2012) Echocardiographic diastolic parameters and risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J 33: 904-912. [Crossref]
- Tsang TS, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME et al. (2002) Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol 40: 1636-1644. [Crossref]
- Vasan RS, Larson MG, Levy D, Galderisi M, Wolf PA et al. (2003) Doppler transmitral flow indexes and risk of atrial fibrillation (the Framingham Heart Study). Am J Cardiol 91: 1079-1083. [Crossref]
- Nayor M, Enserro DM, Xanthakis V, Larson MG, Benjamin EJ et al. (2018) Comorbidities and cardiometabolic disease relationship with longitudinal changes in diastolic function. JACC: Heart Failure 6: 317-325. [Crossref]
- Cioffi G, Giorda CB, Chinali M, Di Lenarda A, Faggiano P et al. (2012) Analysis of mid-wall shortening reveals high prevalence of left ventricular myocardial dysfunction in patients with diabetes mellitus: the DYDA study. Eur J Prev Cardiol 19: 935-943. [Crossref]
- Gong HP, Tan HW, Fang NN, Song T, Li SH et al. (2009) Impaired left ventricular systolic and diastolic function in patients with metabolic syndrome as assessed by strain and strain rate imaging. Diabetes Res Clin Pract 83: 300-307. [Crossref]
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 17: 1321-1360. [Crossref]
- Almeida JG, Fontes Carvalho R, Sampaio F et al. (2018) Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J Cardiovasc Imaging 19: 380-386. [Crossref]
- Gopalakrishnan PP, Biederman R (2020) Impact of the 2016 ASE/EACVI Guidelines on diastolic function reporting in routine clinical practice. Echocardiography 37: 546-553. [Crossref]
- Nayor M, Cooper LL, Enserro DM, Xanthakis V, Larson MG et al. (2018) Left Ventricular Diastolic Dysfunction in the community: Impact of diagnostic criteria on the burden, correlates, and prognosis. J Am Heart Assoc 7: e008291. [Crossref]
- Lin FY, Zemedkun M, Dunning A, Gomez M, Labounty TM et al. (2013) Extent and severity of coronary artery disease by coronary CT angiography is associated with elevated left ventricular diastolic pressures and worsening diastolic function. J Cardiovasc Comput Tomogr 7: 289-296. [Crossref]
- Wachtell K, Bella JN, Rokkedal J, Aurigemma GP, Papademetriou V et al. (2002) Change in diastolic left ventricular filling after one year of antihypertensive treatment: The Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Circulation 105: 1071-1076. [Crossref]
- Yip GW, Wang M, Wang T, Chan S, Fung JW et al. (2008) The Hong Kong diastolic heart failure study: a randomized controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart 94: 573-580. [Crossref]
- Müller Brunotte R, Kahan T, Malmqvist K, Ring M, Edner M (2006) Tissue velocity echocardiography shows early improvement in diastolic function with irbesartan and atenolol therapy in patients with hypertensive left ventricular hypertrophy. Results from the Swedish irbesartan left ventricular hypertrophy investigation vs atenolol (SILVHIA). Am J Hypertens 19: 927-936. [Crossref]
- Terpstra WF, May JF, Smit AJ, de Graeff PA, Havinga TK et al. (2001) Long-term effects of amlodipine and lisinopril on left ventricular mass and diastolic function in elderly, previously untreated hypertensive patients: the ELVERA trial. J Hypertens 19: 303-309. [Crossref]
- Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL et al. (2007) Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomized trial. Lancet 369: 2079-2087. [Crossref]
- ADVANCE Echocardiography Substudy Investigators and the ADVANCE Collaborative Group (2011) Effects of perindopril-indapamide on left ventricular diastolic function and mass in patients with type 2 diabetes: the ADVANCE Echocardiography Substudy. J Hypertens 29: 1439-1447. [Crossref]
- Lan N, Fegan PG, Yeap BB, Dwivedi G (2019) The effects of sodium-glucose cotransporter 2 inhibitors on left ventricular function: current evidence and future directions. ESC Heart Fail 6: 927-935. [Crossref]
- Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL et al. (2009) Cardiac dysfunction assessed by echocardiographic tissue doppler imaging is an independent predictor of mortality in the general population. Circulation 119: 2679-2685. [Crossref]
- Kuznetsova T, Thijs L, Knez J, Herbots L, Zhang Z et al. (2014) Prognostic value of left ventricular diastolic dysfunction in a general population. J Am Heart Assoc 3: e000789. [Crossref]
- Sharp AS, Tapp RJ, Thom SA, Francis DP, Hughes AD et al. (2010) ASCOT Investigators. Tissue Doppler E/E’ ratio is a powerful predictor of primary cardiac events in a hypertensive population: an ASCOT substudy. Eur Heart J 31: 747-752. [Crossref]
- Blomstrand P, Engvall M, Festin K, Lindström T, Länne T et al. (2015) Left ventricular diastolic function, assessed by echocardiography and tissue Doppler imaging, is a strong predictor of cardiovascular events, superior to global left ventricular longitudinal strain, in patients with type 2 diabetes. Eur Heart J Cardiovasc Imaging 16: 1000-1007. [Crossref]
- From AM, Scott CG, Chen HH (2010) The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction: a population-based study. J Am Coll Cardiol 55: 300-305. [Crossref]
- Shah AM, Cikes M, Prasad N, Li G, Getchevski S et al. (2019) Echocardiographic Features of Patients with Heart Failure and Preserved Left Ventricular Ejection Fraction. Am Coll Cardiol 74: 2858-2873. [Crossref]
