Long Term Survival of a Patient with Metastatic Rectal Cancer Treated with Oral Regorafenib - A Case Report
A B S T R A C T
A 56-years-old female had a history of radical proctectomy for carcinoma of rectum on 2003/4/29. Pathology report was Dukes’ C adenocarcinoma with 12 of 24 lymph node showing metastasis. She was managed to have six months of adjuvant chemotherapy of 5- fluorouracil with leucovorin. Computed Tomography (CT) scan on 2013/4/16 was reported as having recurrent tumor in left presacral region with. associated left hydronephrosis and hydroureter. 5400 cGy of radiotherapy was given. CT scan on 2013/8/14 was reported as decreased size of recurrent tumor in left presacral region as compared to last CT with persistent left hydronephrosis and hydroureter due to tumor invasion of middle left ureter. She was then arranged to have chemotherapy of capecitabine, irinotecan oxaliplatin, uracil-futrafur with bevacizumab and Ziv-Aflibercept. Above knee amputation of left leg was performed on 2016/3/29 following poor result of fasciectomy for necrotizing fasciitis. CT scan on 2016/6/6 was reported as interval stable of presacral and left pelvic wall soft tissue mass with calcification, associated left hydronephrosis and hydroureter. 160 mg per day of regorafenib was started from 2016/7/14. She was taking regorafenib regularly in the past three years and 6 months with stable disease. Her last CT scan on 2019/12/27 was reported as stationary appearance of the calcified soft tissue lesion in left presacral region with ipsilateral hydronephroureter and obliteration ipsilateral common iliac vein with prominent venous collaterals in anterior wall of pelvis and with mild left thigh edematous change.
Keywords
Adenocarcinoma, computed tomography, presacral, regorafenib
Introduction
Regorafenib is an oral multikinase inhibitor targeting multiple tumor pathways [1]. Treatment with regorafenib resulted in a 50.6% reduction in the risk of progression or death over placebo in patients with metastatic colorectal cancer [2]. We have experienced a patient with metastatic rectal cancer having long term survival with continuous use of regorafenib. We believe 42-month progression free survival with continuous use of regorafenib is the longest survival reported in the literature.
Case Report
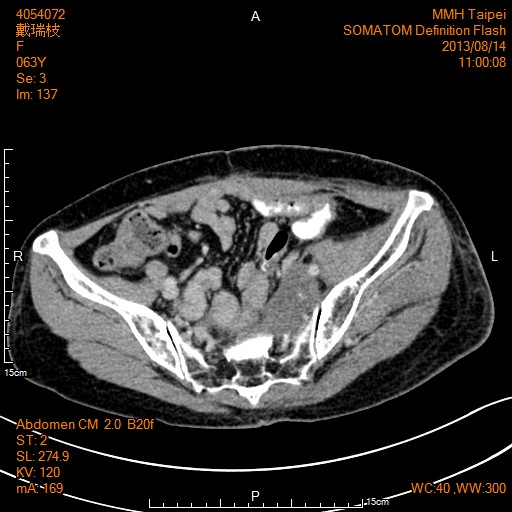
A 56 years old female had a history of radical proctectomy for carcinoma of rectum on 2003/4/29. Pathology report was Dukes’ C adenocarcinoma with 12 of 24 lymph node showing metastasis. Because of endemic of SARS (severe adult respiratory syndrome), she was quarantined a few times because of fever. From 2003/5/22, she was managed to have six months of adjuvant chemotherapy of 5- fluorouracil with leucovorin. Subsequently she had another surgery of low anterior resection for colocutaneous fistula on 2003/12/22. Computed Tomogram (CT) scan on 2013/4/16 was reported as having “Recurrent tumor in left presacral region with size of 3.7 x 6.7 cm, tumor extension to adjacent left sacral foramen is evident. Associated left hydronephrosis and hydroureter are evident, atrophic left kidney is also seen” (Figure 1).
Figure 1: CT scan on 2013/4/16 showed recurrent tumor in left presacral region with size of 3.7 x 6.7 cm, tumor extension to adjacent left sacral foramen is evident.
5400 cGy of radiotherapy with uracil-tegafur and leucovorin were given for the pelvic recurrence, started from 2013/4/29 and finished on 2013/5/22. CT scan on 2013/8/14 was reported as “Decreased size of recurrent tumor in left presacral region as compared to last CT with persistent left hydronephrosis and hydroureter due to tumor invasion of middle left ureter” (Figure 2). She was then arranged to have chemotherapy of capecitabine, irinotecan, oxalipaltin and bevacizumab. Xeloda was switched to UFUR and leucovorin later because of moderate hand and foot syndrome and severe pain of the foot. Bevacizumab was also switched to Ziv-Aflibercept (Zaltrap) later because of insurance problem. Left lower leg fasciectomy was performed on 2016/3/3 and left upper leg fasciotomy was performed on 2016/3/23 for necrotizing fasciitis. And above knee amputation of left leg was performed eventually on 2016/3/29 for necrotizing fasciitis. Cetuximub was not used because of presence of K-ras missense mutation involving codon 13.
Figure 2: CT scan on 2013/8/14 showed decreased size of recurrent tumor in left presacral region.
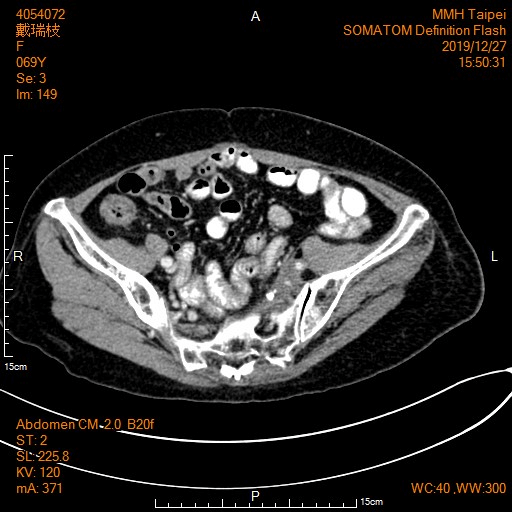
CT scan on 2016/6/6 was reported as “Interval stable of presacral and left pelvic wall soft tissue mass with calcification, associated left hydronephrosis and hydroureter” (Figure 3). 160 mg per day of regorafenib was started from 2016/7/14, although dose of regorafenib was cut down to 120 mg per day because of transit abnormality of liver function and moderate hand and foot syndrome. She was taking regorafenib regularly with three weeks on and one week off schedule and followed closely in the past three years and six months with CT scan for stable disease. Her last CT scan on 2019/12/27 was reported as “Stationary appearance of the calcified soft tissue lesion in left presacral region with ipsilateral hydronephroureter and obliteration ipsilateral common iliac vein with prominent venous collaterals in anterior wall of pelvis and with mild left thigh edematous change” (Figure 4).
Figure 3: CT scan on 2016/6/6 showed interval stable of presacral and left pelvic wall soft tissue mass with calcification.
Figure 4: CT scan on 2019/12/27 showed stationary appearance of the calcified soft tissue lesion in left presacral region.
Discussion
VEGF is a key mediator of angiogenesis in normal tissues and binds two VEGF receptors (VEGF receptor-1 and VEGF receptor-2), which are expressed on vascular endothelial cells [1]. VEGF is also thought to be a key mediator of angiogenesis in cancer [2]. Regorafenib (BAY 73-4506) is an oral multikinase inhibitor targeting multiple tumor pathways1-3. It showed Inhibition of proliferation of tumor cell through biochemical activity of KIT, PDGFR, RET; Inhibition of tumor microenvironment signaling through biochemical activity of PDGFR-β, FGFR; Inhibition of neoangiogenesis is through biochemical activity of VEGFR1-3, TIE2 [3-6].
A CORRECT study was designed to patients with metastatic colorectal cancer treated with regorafenib or placebo after failure of standard therapy. The result showed that OS rates were consistently higher in the regorafenib arm than in the placebo arm at 6- and 12-months post-randomization. Treatment with regorafenib resulted in a 21% reduction in the risk of death over placebo. Regorafenib together with basic support care significantly improved progression-free survival (PFS) and time to progression over placebo with basic support care. Treatment with regorafenib resulted in a 50.6% reduction in the risk of progression or death over placebo [2, 3]. Frequently seen adverse event of regorafenib include hand-foot skin reaction, fatigue, hypertension, diarrhea, and rash/desquamation. In the CORRECT trial, the median duration of treatment was 1.7 months (interquartile range [IQR] 1.4–3.7) [2].
Complete responses were not reported, it is understandable that not many patients would have chance of long-term survival following usage of regorafenib since regorafenib is a salvage therapy for the patients with colorectal cancer after multiple lines of chemotherapy. We hypothesize that either this patient’s tumor was extremely sensitive to the drug and/or that he had favorable tumor biology, characteristics which might explain her long-term survival after the diagnosis of metastatic disease [7].
Since regorafenib also have biochemical activity of VEGFR1-3, rational thought would be it might affect bowel blood supply in some extent. Our patient had an episode of necrotizing fasciitis could be secondary to ischemic change of VEGF.
Conclusion
Whether regorafenib is going to cure some patients with metastatic carcinoma of colorectum is not known, pending on further study. However, aggressive treatment with continuing use of regorafenib is best for the patient’s benefit.
Article Info
Article Type
Case ReportPublication history
Received: Tue 03, Mar 2020Accepted: Thu 02, Apr 2020
Published: Fri 10, Apr 2020
Copyright
© 2023 Tzu-Chi Hsu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.03.10
Author Info
Ming-jen Chen Tzu-Chi Hsu Wen-chun Sun
Corresponding Author
Tzu-Chi HsuDivision of Colon and Rectal Surgery, Department of Surgery, Taipei Mackay Memorial Hospital, Taipei, Taiwan
Figures & Tables


References
- Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69: 4-10. [Crossref]
- Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A et al. (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381: 303-312. [Crossref]
- Li J, Qin S, Xu R, Yau TC, Ma B et al. (2015) Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 16: 619-629. [Crossref]
- Verhoef C, de Wilt JH, Verheul HM (2006) Angiogenesis inhibitors: Perspectives for medical, surgical and radiation oncology. Curr Pharm Des 12: 2623-2630. [Crossref]
- Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA et al. (2011) Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 129: 245-255. [Crossref]
- Strumber D, Schultheis B (2012) Regorafenib for cancer. Expert Opin Investig Drugs 21: 879-889. [Crossref]
- Amram MR, Montet X, Roth AD (2017) Long-Term Survival with Regorafenib in KRAS-Mutated Metastatic Rectal Cancer. Case Rep Oncol 10: 1029-1034. [Crossref]
