Cyclophosphamide, Fluorouracil and subcutaneous Interleukin-2 in the treatment of advanced GIST: A Case Report
A B S T R A C T
A male 68 years hold patient was admitted to surgical ward for hemorrhagic shock. After CT scan detection of 6x5 cm neoformation of first jejunal loop, he was submitted to segmental resection and pathological diagnosis was gastrointestinal stromal tumor. The patient was defined as high-risk according to Takahashi criteria, but refused Imatinib adjuvant therapy. After 15 months of disease-free interval, he developed bilobar liver metastases. After treatment with Imatinib 400 mg he reported G3 hepatotoxicity resolved with temporary suspension, he continue low dose with stable disease. After liver progression, he resumed Imatinib full dose with disease stabilization for 9 months. After liver progression, second line Sunitinib 37,5 mg/day was started for four months with stable disease. After further liver and lymph node mediastinal progression he was treated for four months with Regorafenib with disease stabilization. Patient developed slow but inexorable progression of liver disease with severe abdominal pain resistant to opioid and was treated with authorized compassionate program comprising Cyclophosphamide 300 mg/sqm and Fluorouracil 500 mg/sqm on day 1 intravenously followed by Interleukin-2 4.5 MUI subcutaneously on days 3–6 and 17–20 every four weeks. After three cycles the patients obtained a relevant subjective improvement with partial response on mediastinal lymph node and liver stabilization. A substantial increase on neutrophil, lymphocytes, monocytes, platelets, T regulator cells count, and a decrease on platelets/lymphocytes, CD8/T regulator cells ratio, CD8, NK count and C-reactive protein value were observed after treatment compared to basal value. The toxicity was mild represented by fever G1, flue-like-syndrome G1 during the treatment. After four cycle of chemo-immunotherapy, the patient demonstrated progression of disease and died five months after treatment. Noteworthy is the temporal disease control with significant symptomatic improvement achieved for the first time with this chemo-immunotherapeutic combination in a patient with very advanced pretreated GIST.
Keywords
Cyclophosphamide, fluorouracil, subcutaneous IL-2, GIST, imatinib
Introduction
The growing interest resulting from the evidence that immune suppression plays an important role in the progression of disease in tumors has aroused much interest in the treatment of resistant tumors. The description of this case of heavily pre-treated GIST pays attention to a combined treatment that confirms the possibility of translating into practice the theoretical assumptions underlying immunosuppression
Case Report
A male 68 years hold patient on December 2010 was admitted to the surgical ward for hemorrhagic shock by neoformation bleeding of the first jejunal loop. The patient underwent esophagogastroduodenoscopy and rectosigmoidoscopy which were negative for neoplastic disease. The CT scan showed 5 cm round oval formation contained in the adipose tissue of proximal jejunal loop. Enhancement of the lesion wall and of the intestinal lumen contiguous to the mass, slightly dissociability from adjacent intestinal loops and no widespread metastatic lesions were detected. On December 2010 the patient was submitted to segmental resection of bleeding of 6x5 cm neoformation of the first jejunal loop. The histopathological specimen showed stromal tumor (CD117 + of membrane and paranuclear cytoplasmic, CD34 + zonal; Caldesmone-, Desmina-, S100 -) infiltrating intestinal muscle tunic, mitotic index of 15/10 HPF 40 x (Ki 67 15-20%), zonal areas of necrosis and hemorrhage. Remaining visceral wall and resection margins were free of neoplastic infiltration. Therefore, for small bowel invasion the patient was defined as high risk according to Takahashi [1].
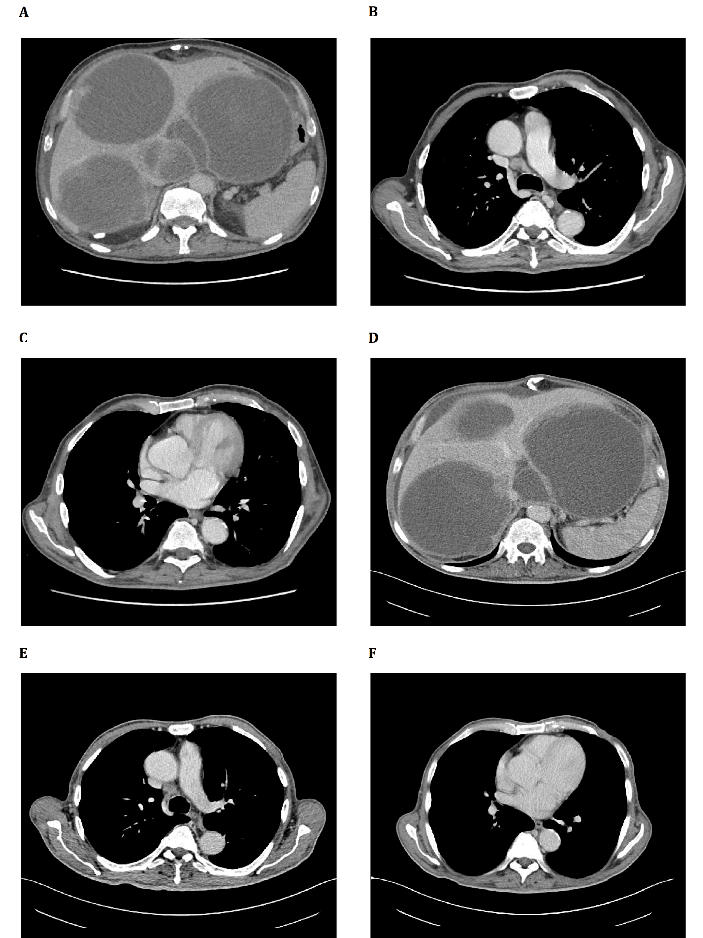
Figure 1A: Increased in number and size the hypodense lesions, the greater to the II segment of 16 cm. The portal vein appears focally compressed by localized I segment formation of 7.5 cm). The inferior vena cava remains thinned but still open, in the retro-hepatic tract. Presence of peri-hepatic fluid effusion
Figure 1B: Slight increase in size of lymph node formation in the Barety loggia (25x14 mm) and in the right hilar area (14x16 mm) (Figure 1C)Figure 1 D) Not substantial variations in number and size but more hypodensity of the metastatic lesions. No new focal lesions in the spleen. Persistence of peri-hepatic fluid effusion
Slightly reduction of the Barety lodge lymph node (21x13 mm) ( Figure 1 E) and the one in the right hilar area (13x7 mm) (Figure 1F)
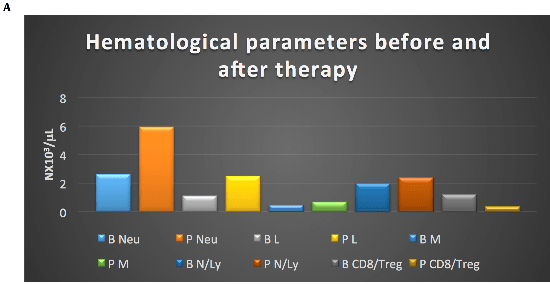
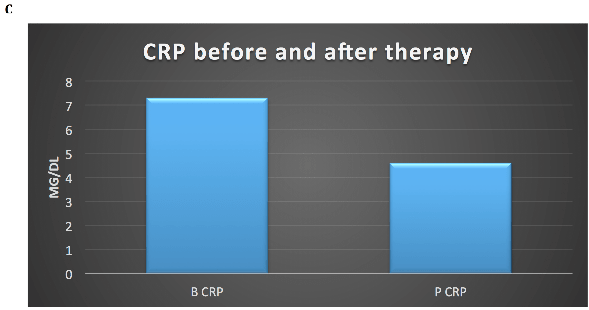
Postoperatively the patient refused Imatinib (IM) adjuvant therapy. After disease-free interval of 15 months he developed bilobar liver metastases with necrotic component in the caudate lobe (40 mm) and VII segment (35 mm). From April 2012 he was treated with IM 400 mg but he reported G3 hepatotoxicity resolved with IM suspension. Thereafter from July 2012 to May 2014 he continues IM 300 mg/day with stable disease. After liver progression, he resumed full dose IM from May 2014 to October 2015 obtaining a disease stabilization for 9 months. After liver progression, from November 2015 to March 2016 Sunitinib 37,5 mg/day was started with stable disease. After further liver and lymph node mediastinal progression he was treated from June 2016 to October 2016 with standard dose of Regorafenib with disease stabilization. Toxicities of TKI inibithors were asthenia G1, dysgeusia, anorexia, epigastralgia, diarrhea G2. After slow but inexorable progression of liver disease (Figure 1 A) with huge, hard consistency, hepatomegaly resulting in severe abdominal pain resistant to opioid therapy and mediastinal lymph node enlargement (Figure1 B-C), he was treated with authorized compassionate program consisting on outpatient Cyclophosphamide (Cyt) 300 mg/sqm and Fluorouracil (FU) 500 mg/sqm on day 1 intravenously followed by Interleukin-2 (IL-2) 4.5 MUI subcutaneously on days 3–6 and 17–20. The cycle was repeated on day 29. The treatment plan provided three cycles of therapy followed by further three cycles if stabilization or objective response [2]. The patients after three cycles of therapy obtained a relevant subjective improvement with liver stabilization (Figure 1 D) and partial response on mediastinal lymph node (Figure 1 E-F). A substantial increase on neutrophil, lymphocytes, monocytes, platelets, Tregs count (Figure 2 A-B), and a decrease on platelets/lymphocytes, CD8/Treg ratio, CD8, NK count (Figure 2 B) and C-reactive protein value (Figure 2 C) were observed after treatment. The toxicity was mild and was represented by fever G1, flue-like-syndrome G1 during the treatment. After four cycle of chemo-immunotherapy, the patient developed progression of disease and died five months after treatment.
Figure 2A: Increase of neutrophil (Neu), lymphocytes (Ly), monocytes (M), N/Ly, decrease of CD8/Ly T regulator (Treg) ratios after therapy; B: basal; P: post-therapy
Figure 2B: Increase of platelets (PLT), lymphocyte T regulator (Treg), decrease of PLT/Lymphocyte (Ly) (PLT/Ly), CD8, and natural killer (NK) count after therapy; B: basal; P: post-therapy
Figure 2C: Decrease of C-Reactive Protein (CRP) after therapy; B: basal; P: post-therapy
Discussion
Metastatic GIST represents a chemo-resistant disease. It was considered an orphan neoplasm until 2000 when IM activity was tested, showing significant efficacy in advanced disease, especially in patients with exon 11 c-Kit mutation [3]. Furthermore, adjuvant IM have positive impact on prognosis and survival in the intermediate-high risk, according to Miettinen prognostic score [4, 5]. However, after disease progression both after IM adjuvant and palliative therapy for advanced disease, different rates of primary or secondary resistance are observed depending on the type of mutation. IM exerted immunomodulatory functions by inhibiting and/or activating specific subsets of immune cells mostly natural killer (NK) and progressive loss of MHC [6-8]. The immune system in GIST presents a dynamic picture with variations on the primary tumor and metastases and in relation to IM treatment and to c-Kit mutation that gives predictive and prognostic value. After the demonstration in the animal model of host dentritic cell (DC) revertant effect on resistance to IM through the promotion of the NK-cell-dependent antitumor effect, subsequent studies demonstrate the CD11c+B220+NK1.1+ cells increase during the combination therapy of IM plus IL-2 for the production IFN-γ by contact with tumor cells naming these CD11c+ cells IFN-producing killer DC (IKDC) [9, 10].
Further experience of Gustave Roussy chemo-immuno-target regimen comprising oral metronomic Cyt combined to IM 400 mg/day from D-21 to D 14 before escalating dose (3, 6, 9 and 12 MIU/day) of subcutaneous IL-2 was done. The study highlighted the feasibility of the treatment, the achievement of maximum tolerated dose and the influence of IL-2 on the increase in concentration and impregnation of IM and its metabolite but without objective response [11]. Furthermore, a positive correlation between IL-2 dose and reduction of the absolute counts of B, CD4+ and CD8+ T cells, a slight increase of regulatory T cells (Tregs) rate and NK cell activation with upregulation of HLA-DR, tumor necrosis factor-related apoptosis-inducing ligand and CD56 has been demonstrated. The overexpression of HLA-DR+ natural killer cell levels were positively associated with progression-free and overall survival. Moreover, the CD4+/CD8+ T-cell ratio increase was predictor of overall survival post all treatment steps [12]. The predictive and prognostic value of CD4/CD8 ratio as of Neutrophil/Lymphocyte ratio (NLR) was reported with sunitinib in advanced GIST and are in agreement with what has been observed in other neoplastic pathologies [13-15]. Recently we have witnessed an explosion concerning the research on new immunotherapeutic agents that have come into clinical practice both in solid and hematological tumors.
However, whereas some efficacy has been found with ipilimumab alone and in combination with nivolumab [16, 17], these results have not been confirmed by with the combination of metronomic Cyt to anti- PD1 Pembrolizumab in pretreated soft tissue sarcoma (STS) patients and GIST, characterized by low expression of PDL-1≥ 1% on immune cells and high expression of CD163-positive macrophages with M2 phenotype, indoleamine 2,3-dioxygenase 1 and its product Kynurenine. These factors play a role in immune suppression and resistance to PD-1/PD-L1 targeting in STS and GIST [18]. In light of the uncertainty on the efficacy data of the new anti-CTL-A4 inhibitors and Anti-PD-1/anti-PDL-1 check-point agents, and the direct and indirect immunosuppression determined by Treg and MDSC, the rediscovery of the effectiveness of IL-2, assisted by immune-modulating agents could be justified. IL-2 is produced by activated T cells and increase the proliferation and activation of cytotoxic T cells, natural killer (NK) cells and monocytes [19]. IL-2 is essential for the maintenance and expansion of immunosuppressive CD4 + CD25 + Tregs, in which IL-2Rαβγ is constitutively expressed, to reduce the risk of an uncontrolled immune responses and autoimmunity [20]. After Food and Drug Administration approval in metastatic renal cancer in 1992 and metastatic melanoma in 1998, it represents the only molecule capable of achieving long-term survival in < 10% of patients with these tumors. It can be used in different doses and modalities of administration, but a high-dose bolus has been shown to be superior to other modalities only in patients attaining complete remission in renal cancer [21]. Furthermore, sporadic activity has been reported in other non-immunogenic tumors [22-24].
Considering the role played by Treg and MDSC on anti-tumor immunosuppression, it is therefore possible to hypothesize that agents capable of negatively modifying these two immunosuppressive cells can improve the antitumor immune activity. In light of the superiority of combination drugs on both immune-suppressive cells [25] we undertook areal-world experience with Cyt alone or in combination with FU, prior to the administration of IL-2, in heavily pre-treated solid tumor patients not susceptible to other potentially effective treatments [2]. From this experience we found a transient decrease of Treg after therapy, especially with intravenous IL-2 administration, a subjective improvement with pain control, objective response on 23% of patients, and a reasonable lengthening in survival. Noteworthy is the temporal disease control with significant symptomatic improvement achieved for the first time with this chemo-immunotherapeutic combination in a patient with very advanced GIST disease. Moreover, the increase and the blood decrease respectively of Treg and NK cells observed in this GIST case could make to hypothesize their opposite representation in the tumor but that should be substantiated from the pathological point of view. However our overall preliminary data require further perspective study in a more selected series with the aim of verifying in a more timely manner the possible temporal variations of lymphocyte subpopulations on the tumoral tissue and on the blood by comparing chemo-immunotherapy with a control group with immunotherapy alone and its correlation with response to treatment and survival.
Conflicts of Interest
None
Acknowledgments
We thank the patient, family, doctors and nurses of medical oncology who have allowed this experience aimed at improving the therapeutic index and quality of life of patient.
Abbreviation
GIST: gastro intestinal stromal tumour; CD117: tyrosine-protein kinase Kit; CD34: transmembrane phosphoglycoprotein; S100: S100 protein; KI 67: KI 67 antigen; Cyt: Cyclophosphamide; FU: Fluorouracil; IL-2: Interleukin-2; HPF: high power fields; IM: Imatinib; Tregs: Lymphocyte T regulator cells; CD8: Lymphocyte T CD8; NK: Natural killer; CRP: C-reactive protein; c-KIT: proto-oncogene tyrosine kinase receptor KIT; MCH: major histocompatibility complex; DC: dentritic cell; STS: soft tissue sarcoma; IFN ϫ: interferon gamma; IKDC: CD11c+ cells IFN-producing killer DC; HLA-DR: MHC class II cell; CD4: Lymphocyte T CD4; CD56: Lymphocyte T CD56; NLR: Neutrophil/Lymphocyte ratio; PD-1: Programmed cell death protein 1; PDL-1: Programmed death-ligand 1; CTLA-4: Cytotoxic T-Lymphocyte Antigen 4; MDSC: myeloid-derived suppressor cells; CD25: Lymphocyte T CD25; IL-2Rαβγ: interleukin-2 receptor αβγ
Article Info
Article Type
Case ReportPublication history
Received: Sun 21, Apr 2019Accepted: Wed 08, May 2019
Published: Sat 29, Jun 2019
Copyright
© 2023 Giovanni Lo Re . This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2019.03.03
Author Info
Alessandro Del Conte Francesco Lo Re Giovanni Lo Re Paolo Doretto Paolo Ubiali Piero Brosolo Sandro Sulfaro Wally Marus
Corresponding Author
Giovanni Lo ReCRO Aviano Medical Oncology and immunerelated tumors, Italy
Figures & Tables

References
- Takahashi T, Nakajim K, Nishitani A, Souma Y, Hirota S et al. (2007) An enhanced risk-group stratification system for more practical prognostication of clinically malignant gastrointestinal stromal tumors. Int J Clin Oncol 12: 369-374. [[Crossref]]
- Lo Re G, Lo Re F, Doretto P, Del Conte A, Amadio M et al. (2019) Cyclophosphamide with or without fluorouracil followed by subcutaneous or intravenous interleukin-2 use in solid tumors: A feasibility off-label experience. Cytokine 113: 50-60. [Crossref]
- Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B et al. (2008) Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 26: 620-625. [Crossref]
- Joensuu H, Eriksson M, Sundby Hall K et al. (2012) Twelve vs. 36 months of adjuvant imatinib as treatment of operable GIST with a high risk of recurrence. Final results of a randomized trial (SSGXVIII/AIO). JAMA 12: 1265-1272.
- Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 23: 70-83. [Crossref]
- Borg C, Terme M, Taïeb J, Menard C, Flament C et al. (2004) Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest 114: 379-388. [Crossref]
- Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S et al. (2011) Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med 17: 700-707. [Crossref]
- Rusakiewicz S, Semeraro M, Sarabi M, Desbois M, Locher C et al. (2013) Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res 73: 3499-3510. [Crossref]
- Borg C, Terme M, Taïeb J, Menard C, Flament C et al. (2004) Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest 114: 379-388. [Crossref]
- Taieb J, Chaput N, Ménard C, Apetoh L, Ullrich E et al. (2006) A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med 12: 214-219. [Crossref]
- Pautier P, Locher C, Robert C, Deroussent A, Flament C et al. (2013) Phase I clinical trial combining imatinib mesylate and IL-2 in refractory cancer patients: IL-2 interferes with the pharmacokinetics of imatinib mesylate. Oncoimmunology 2: e23079. [Crossref]
- Chaput N, Flament C, Locher C, Desbois M, Rey A et al. (2013) Phase I clinical trial combining imatinib mesylate and IL-2: HLA-DR+ NK cell levels correlate with disease outcome. Oncoimmunology 2: e23080. [Crossref]
- Rutkowski P, Lugowska I, Klimczak A (2018) The prognostic value of blood neutrophil-to-lymphocyte ratio (NLR) factor in advanced gastrointestinal stromal tumors (GIST) treated with sunitinib after imatinib failure J Clin Oncol 36 .
- Shah W, Yan X, Jing L, Zhou Y, Chen H et al. (2011) A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4(+) FOXP3(+) regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell Mol Immunol 8: 59-66. [Crossref]
- Azad Gazi Sahin, Cengiz Aydin, Mutlu Unver, Kamil Pehivanoglu (2017) Predictive value of Preoperative Neutrophil Lymphocyte Ratio in Determining the Stage of Gastric Tumor. Med. Sci. Monit 23: 1973-1979. [Crossref]
- Reilley MJ, Bailey A, Subbiah V, Janku F, Naing A et al. (2017) Phase I clinical trial of combination imatinib and ipilimumab in patients with advanced malignancies. J Immunother Cancer 5: 35. [Crossref]
- Singh AS, Chmielowski B, Hecht JR (2018) A randomized phase 2 study of nivolumab monotherapy versus nivolumab combined with ipilimumab in patients with metastatic or unresectable gastrointestinal stromal tumor. NCT02880020. J Clin Oncol 36.
- Toulmonde M, Penel N, Adam J, Chevreau C, Blay JY et al. (2017) Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas A Phase 2 Clinical Trial. JAMA Oncol 4: 93-97. [Crossref]
- Rosenberg SA, Mulé JJ, Spiess PJ, Reichert CM, Schwarz SL et al. (1985) Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med 161: 1169-1688. [Crossref]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY et al. (2005) A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 6: 1142-1151. [Crossref]
- Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ et al. (2003) Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 21: 3127-3132. [Crossref]
- Hayakawa K, Fukushima T, Seito D, Asakura Y, Yamanaka Y et al. (1989) Continuous arterial infusion therapy of recombinant interleukin-2 and cyclophosphamide in hepatoma and liver metastasis. Gan To Kagaku Ryoho 16: 2842-2844. [Crossref]
- Mayer N. Fishman, Daniel A. Vaena (2015) Phase Ib/II study of an IL-2/T-cell receptor fusion protein in combination with gemcitabine and cisplatin in advanced or metastatic chemo-refractory urothelial cancer (UC). J Clin Oncol 33.
- Wei S, Kryczek I, Edwards RP, Zou L, Szelige W et al. (2007) Interleukin-2 administration alters the CD4+FOXP3+ T-cell pool and tumor trafficking in patients with ovarian carcinoma. Cancer Res 67: 7487-7494. [Crossref]
- Tongu M, Harashima N, Monma H, Inao T, Yamada T et al. (2013) Metronomic chemotherapy with low-dose cyclophosphamide plus gemcitabine can induce anti-tumor T cell immunity in vivo. Cancer Immunol Immunother 62: 383-391. [Crossref]
